Maureen E. Hill/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
The <scene name='56/566502/Active_site_conformation/1'>active site</scene> is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the <scene name='56/566502/Procaspase_zymogen/1'>procaspase-7 zymogen</scene>, the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. <scene name='56/566502/Active_site_substrate_color/1'>Caspase-7 bound to suicide inhibitor/substrate mimc DEVD-CHO</scene> traps the protein an active/substrate bound conformation. Substrate binding forces a conformational change moving L2' upward, this creates a foundation beneath the L2 bundle stabilizing the active complex. | The <scene name='56/566502/Active_site_conformation/1'>active site</scene> is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the <scene name='56/566502/Procaspase_zymogen/1'>procaspase-7 zymogen</scene>, the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. <scene name='56/566502/Active_site_substrate_color/1'>Caspase-7 bound to suicide inhibitor/substrate mimc DEVD-CHO</scene> traps the protein an active/substrate bound conformation. Substrate binding forces a conformational change moving L2' upward, this creates a foundation beneath the L2 bundle stabilizing the active complex. | ||
| + | |||
| + | Mutagenesis performed within this region of the protein has a significant impacted on the ability of the protein to process its substrates. Ultimately, this confirms the importance of L2' stabilizing the active site loop bundle. | ||
== Allosteric Inhibition of Caspase-7 == | == Allosteric Inhibition of Caspase-7 == | ||
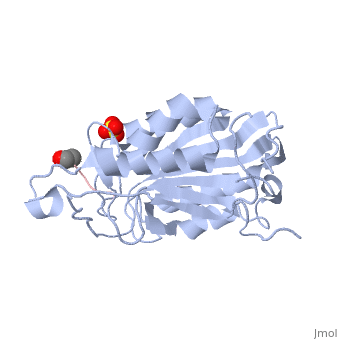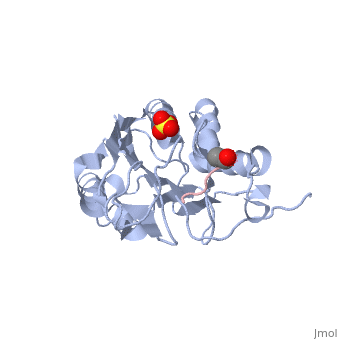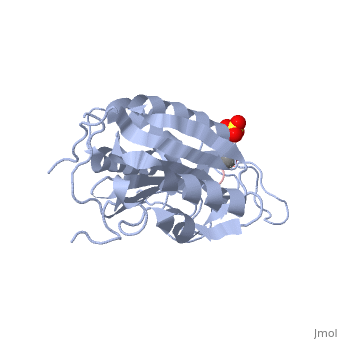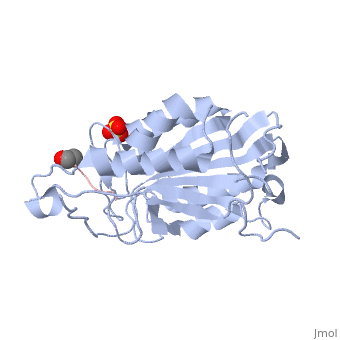
| - | It has been shown that caspases -3 and -7 can be inhibited at a site other than the active site by allosteric inhibitors, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide ('''FICA''') or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide ('''DICA'''), at the dimer-interface cavity. | + | It has been shown that caspases -3 and -7 can be inhibited at a site other than the active site by allosteric inhibitors, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide ('''FICA''') or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide ('''DICA'''), at the dimer-interface cavity. These inhibitors inactivate the enzyme on three different levels by: A) locking the L2' in a down conformation preventing it from ordering the active site loop bundles, B) rearrangement of the active site dyad H144 and C186, movements within the active site affect the ability to perform its chemistry, C) a conformational shift of residue R187 blocks substrate binding. |
| - | <scene name='56/566502/Dica_bound_caspase_7/2'>Caspase-7 bound to allosteric inhibitor DICA</scene> at the dimer interface. The allosteric | + | <scene name='56/566502/Dica_bound_caspase_7/2'>Caspase-7 bound to allosteric inhibitor DICA</scene> at the dimer interface. The mechanism of allosteric inhibition of DICA starts with binding to C290 within the dimer interface, this displaces Y223. The displacement of tyrosine from the active site conformation of the enzyme forces R187 into a position that both physically blocks substrate binding, as well as, move the active site cystine 186. Ultimately, these conformational changes inactivate the enzyme. |
Revision as of 16:01, 3 December 2013
Caspases are a family of CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Executioner Caspase-7
| |||||||||||