Background
Caspases are cysteine-aspartate proteases that are responsible for the execution of apoptosis, also known as programmed cell death. Dysregulation of apoptosis has been linked to neurodegenerative disorders, including Alzheimer's and Huntington's, as well as inflammatory diseases and cancer.
The apoptotic caspases consist of two distinct classes: the initiators (caspase -2, -8, -9, and -10) and the executioners (caspase -3, -6, and -7). All caspases are synthesized as catalytically inactive zymogens that must undergo proteolytic cleavage to be activated during apoptosis. Initiator caspases are activated by upstream cellular events, which in turn cleave at distinct internal aspartate residues in the executioner caspases to remove the prodomain and separate the large and small subunits. The executioner caspases then cleave a wide range of targets within the cell that ultimately leads to cellular suicide.
Caspase-7 Structure
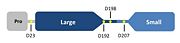
Cleavage sites of caspase-7
Caspases are crystallized as homodimers. As previously stated, the caspases undergo proteolytic cleavage by the initiator caspases to assume their active conformations. Some caspases undergo more cleavage than others. Caspase-7 has three major cleavage sites: D23, D198 and D207. D23 processing removes the prodomain from the large subunit, whereas D198 and D207 are the major cleavage sites for processing and removal of the inter-subunit linker. Caspase-7 has one minor cleavage site also located within the inter-subunit linker at D192.
The is made up of four flexible loops which include L2, L3 and L4 from one half of the dimer that interact with L2' from the opposite half of the dimer. In the , the loops are disordered, which prevents substrate binding. Upon cleavage at the intersubunit linker, the active-site loop bundle becomes partially ordered, whereas L2' stays in the inactive, down conformation. At this point, caspase-7 may bind either substrate or allosteric inhibitors. traps the protein an active/substrate bound conformation. Substrate binding forces a conformational change moving L2' upward, this creates a foundation beneath the L2 bundle stabilizing the active complex. Mutagenesis performed within this region of the protein has a significant impacted on the ability of the protein to process its substrates. Ultimately, this confirms the importance of L2' stabilizing the active site loop bundle.
Allosteric Inhibition of Caspase-7
It has been shown that caspases -3 and -7 can be inhibited at a site other than the active site by allosteric inhibitors, such as 5-Fluoro-1H-indole-2-carboxylic acid (2-mercapto-ethyl)-amide (FICA) or 2-(2,4-Dichlorophenoxy)-N-(2-mercapto-ethyl)-acetamide (DICA), at the dimer-interface cavity. These inhibitors inactivate the enzyme on three different levels by: A) locking the L2' in a down conformation preventing it from ordering the active site loop bundles, B) rearrangement of the active site dyad H144 and C186, movements within the active site affect the ability to perform its chemistry, C) a conformational shift of residue R187 blocks substrate binding.
at the dimer interface. The mechanism of allosteric inhibition of DICA starts with binding to C290 within the dimer interface, this displaces Y223. The movement of tyrosine from the partially active state of the enzyme forces R187 into a position that both physically blocks substrate binding, as well as, move the active site cysteine 186. The resulting conformational changes inactivate the enzyme, making it unable to process substrate.
Caspase-7 Dynamics
Observed here is the of Caspase-7. These dynamics show the dramatic conformational change of the cleaved semi-ordered protease upon binding to the substrate mimic DEVD-CHO. Substrate binding triggers rearrangement of the partially ordered loop bundles and the L2' loop. The newly assumed state stabilizes the protein.
References
Hardy, J. A., J. Lam, et al. (2004). "Discovery of an allosteric site in the caspases." Proc Natl Acad Sci U S A 101(34): 12461-12466.
Witkowski, W. a, & Hardy, J. a. (2009). L2’ loop is critical for caspase-7 active site formation. Protein science : a publication of the Protein Society, 18(7), 1459–68. doi:10.1002/pro.151
Witkowski, W. a, & Hardy, J. a. (2011). A designed redox-controlled caspase. Protein science : a publication of the Protein Society, 20(8), 1421–31. doi:10.1002/pro.673
