Sandbox Reserved 774
From Proteopedia
| Line 12: | Line 12: | ||
Has a chain structure with 2.4 A resolution, and 2.9 A resolution with a co-factor (acetyl-CoA).<ref name=Shiva/> The method used to determine the structure was [[X-ray crystallography]]. Sedimentation and crystal structure analysis clearly shows that Hpa2 is dimeric in solution and tetramerizes in the unit crystal. The crystal structure of the oligomer reveals that two Hpa2 dimers are held together by interaction between the bound acetyl-CoA molecules. The average B-factor value is 23.9 (<scene name='56/564050/Bakhbone_mainechain/1'>main chain</scene>) with a 25.4 <scene name='56/564050/Sidechain/2'>side chain</scene>. The R-factor is 0.19. <ref name=Shiva/> Core fold features include four conserved sequence motifs of the GNAT family and comprises a central highly curved five stranded <scene name='56/564050/Beta_sheets/1'>Beta sheets</scene> (β1-β5) surrounded on both sides by helical segments (α1 and α3).<ref name=Shiva/> | Has a chain structure with 2.4 A resolution, and 2.9 A resolution with a co-factor (acetyl-CoA).<ref name=Shiva/> The method used to determine the structure was [[X-ray crystallography]]. Sedimentation and crystal structure analysis clearly shows that Hpa2 is dimeric in solution and tetramerizes in the unit crystal. The crystal structure of the oligomer reveals that two Hpa2 dimers are held together by interaction between the bound acetyl-CoA molecules. The average B-factor value is 23.9 (<scene name='56/564050/Bakhbone_mainechain/1'>main chain</scene>) with a 25.4 <scene name='56/564050/Sidechain/2'>side chain</scene>. The R-factor is 0.19. <ref name=Shiva/> Core fold features include four conserved sequence motifs of the GNAT family and comprises a central highly curved five stranded <scene name='56/564050/Beta_sheets/1'>Beta sheets</scene> (β1-β5) surrounded on both sides by helical segments (α1 and α3).<ref name=Shiva/> | ||
| + | |||
| + | <Structure load='1QSM' size='300' frame='true' align='right' caption='3D Model of Cofactor' scene='Insert optional scene name here' /> | ||
=Secondary Structure= | =Secondary Structure= | ||
Revision as of 20:21, 3 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Contents |
Histone Acetyltransferase Hpa2
Hpa2 is a member of the GNAT (Gcn5-related N-acetyltransferases) super-family of enzymes that are found spread out across nature and use acyl-CoA's to acylate their cognate substrates.[1] Histone Acetyltransferase Hpa2 is found in the organism Saccharomyces Cerevisiae, which is more commonly known as Baker's Yeast.[2] In vitro, Hpa2 serves to acetylate histone H3 'Lys-4' and 'Lys-14' and histone H4 'Lys-5' and 'Lys-12.' In solution, Hpa2 forms a dimer, and upon binding with AcCoA forms a tetramer.[1][3] It is classified as a transferase.[3]
Structure
Has a chain structure with 2.4 A resolution, and 2.9 A resolution with a co-factor (acetyl-CoA).[3] The method used to determine the structure was X-ray crystallography. Sedimentation and crystal structure analysis clearly shows that Hpa2 is dimeric in solution and tetramerizes in the unit crystal. The crystal structure of the oligomer reveals that two Hpa2 dimers are held together by interaction between the bound acetyl-CoA molecules. The average B-factor value is 23.9 () with a 25.4 . The R-factor is 0.19. [3] Core fold features include four conserved sequence motifs of the GNAT family and comprises a central highly curved five stranded (β1-β5) surrounded on both sides by helical segments (α1 and α3).[3]
|
Secondary Structure
Most of the secondary structure elements of the monomer contribute residues involved in dimer contacts. A large part of the interface is formed by two projections from the core part of the monomer structure. The first projection is formed by the C-terminal end of strand B3, turn B3-B4, and the N-terminal end of strand B4, while the second is formed by strand B7. Together with strands B5 and B6 they form a barrel-like structure containing ten strands in which the component strands of the barrel locked together.[3] In each structure there are several multi-chain carbonyl groups without hydrogen bonding partners in the active site. These could act in a proton transfer pathway by helping locate water molecules. Around the acetyl group, there exists a hydrophobic pocket which would stabilize the neutral charge while the substrate is bound to the enzyme once the amino group is deprotonated.
Kinetic Mechanism
The binding of substrates and release of products can be random, fully ordered, or a combination of both. It operates on a Bi-Bi mechanism. A study that employed product inhibitors CoA and acetylated (Lys14Ac) H3 peptide and dead-end inhibitor desulfo-CoA in order to determine the order of substrate binding has yielded results consistent with a fully ordered Bi-Bi kinetic mechanism where AcCoA is the first substrate to bind, and CoA is the last product that is released. It is also important to note that the transcriptional co-activator GCN5 from yeast (yGCN5) is a histone acetyltransferase that is essential for the activation of target genes. Bi-substrate kinetic analysis using acetyl-coenzyme A and an H3 histone synthetic peptide indicated that both substrates must bind to form a ternary complex before catalysis. Product inhibition studies revealed that the product CoA was a competitive inhibitor as opposed to AcCoA. Desulfo-CoA, a dead end inhibitor, also demonstrated simple competitive inhibition versus AcCoA. Acetylated (Lys14Ac) H3 peptide displayed noncompetitive inhibition against both H3 peptide and AcCoA.[4]
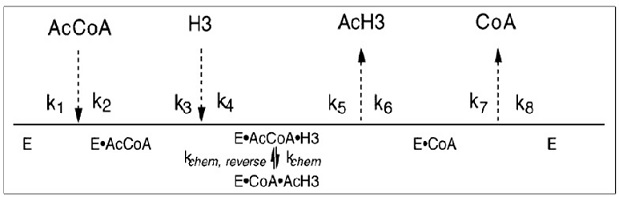
Chemical Mechanism
Post-translational modification of histones is linked to numerous cellular processes. These include transcriptional regulation, DNA damage repair, and DNA replication. One common histone modification, N-e-lysine acetylation, is controlled by the opposing actions of histone acetyltransferases (HATs) and deacetylases (HDACs). After the formation of a ternary complex of acetyl-CoA, histone and enzyme, an active site base deprotonates lysin, which allows for direct attack of the N-e-lysine on the carbonyl carbon of acetyl-CoA. Additionally, without a histone acceptor, slow rates of enzyme auto-acetylation (7 x 10-4 s-1, or ~2500-fold slower than histone acetylation; kcat = 1.6 s-1) and of CoA formation (0.0021 s-1) were not consistent with a kinetically competent acetyl-enzyme intermediate.[5]
Implications
The analysis of a loss-of-function mutant of the hpa2 gene suggests that the hpa2 affects bacterial proliferation in host plants and a hypersensitive response in nonhost plants. As this is the first of such enzyme activity identified in the Hrp protein family, we speculate that the Hpa2 contributes to the assembly of the TTSS by enlarging gaps in the peptidoglycan meshwork of bacterial cell walls.[6] Hpa2 also plays a role in governing gene expression through its effects on chromatin structure and assembly. Additionally, it affects gene regulation, antibiotic resistance, and hormonal regulation of circadian rhythms.
References
- ↑ 1.0 1.1 "Histone Acetyltransferase HPA2 from Saccharomyces Cerevisiae." Protein Data Bank. EMDataBank, n.d. Web. 17 Nov. 2013.RCSB.org
- ↑ "Q06592 (HPA2_YEAST) Reviewed, UniProtKB/Swiss-Prot." Unitprot.org. UniProtKB, 13 Nov. 2013. Web. 16 Nov. 2013.UnitPro.org
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Angus-Hill, et al. "Crystal Structure of the Histone Acetyltransferase Hpa2: a Tetrameric Member of the Gcn5-related N-acetyltransferase Superfamily." J. Mol. Biol. 1999.3338 (1999): 1-14. Web. 18 Nov. 2013.
- ↑ Tanner, et al. "Kinetic Mechanism of the Histone Acetyltransferase GCN5 from Yeast." J. Biol. Chem. 275.29 (2000): 2-9. Web. 26 Nov. 2013.
- ↑ Berndsen, et al. "Catalytic Mechanism of a MYST Family Histone Acetyltransferase." American Chemical Society 46.3 (2007): 1-5. Web. 26 Nov. 2013.
- ↑ Zhang, et al. "A Conserved Hpa2 Protein Has Lytic Activity Against the Bacterial Cell Wall in Phytopathogenic Xanthomonas Oryzae." Appl. Microbiol. Biotechnology 79.4 (2008): 1. Web. 19 Nov. 2013. NCBI.nlm.nih.gov
