Sandbox Reserved 766
From Proteopedia
(→Structure) |
|||
| Line 9: | Line 9: | ||
== Structure == | == Structure == | ||
| - | + | '''Asparagine Synthetase''' is a homodimer comprised of two identical domains ligated together. | |
== Function == | == Function == | ||
Revision as of 04:04, 4 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Introduction
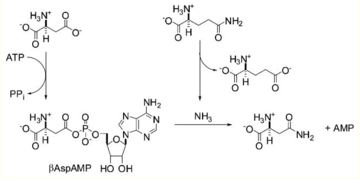
Asparagine Synthetase (ASNS) is an enzyme that catalyzes the conversion of Aspartic Acid to Asparagine through an ATP dependent amination reaction. ASNS can be found in both plants and mammals, however, in plants there are two forms ASNS-A and ASNS-B. Both forms use the same starting molecule of Aspartic Acid and produce Asparagine, the only difference coming from the source of the Nitrogen. ASNS-A uses inorganic Nitrogen in the form of N2 and ASNS-B uses Glutamine as its Nitrogen source; ASNS-B will be the focus of this page because it is very similar and undergoes the same reaction as ASNS in mammals.
Structure
Asparagine Synthetase is a homodimer comprised of two identical domains ligated together.
Function
Asparagine Synthetase catalyzes the interconversion of Aspartic Acid and Glutamine to Asparagine and Glutamic Acid. This is not a straight forward transferase reaction since the interconversion is not directly between Aspartic Acid and Glutamine. Instead this reaction more closely resembles a two part ATP dependent ligase reaction.