Sandbox Reserved 763
From Proteopedia
| Line 13: | Line 13: | ||
==Structure== | ==Structure== | ||
The crystal structure of [[2iyq]] from the Protein Data Bank <ref name="PDB">Protein Data Bank http://www.rcsb.org/pdb/explore.do?structureId=2IYQ</ref> showing shikimate kinase from ''Mycobacterium tuberculosis'' complexed with ADP and shikimate is shown to the right as the <scene name='56/564039/Defaultscene/1'>default scene</scene>. In addition, the 2D model of [[2gij]], showing the space filling details of the asymmetric unit of MtSK, is shown to the left. | The crystal structure of [[2iyq]] from the Protein Data Bank <ref name="PDB">Protein Data Bank http://www.rcsb.org/pdb/explore.do?structureId=2IYQ</ref> showing shikimate kinase from ''Mycobacterium tuberculosis'' complexed with ADP and shikimate is shown to the right as the <scene name='56/564039/Defaultscene/1'>default scene</scene>. In addition, the 2D model of [[2gij]], showing the space filling details of the asymmetric unit of MtSK, is shown to the left. | ||
| - | |||
===3D Structures in Different Organisms=== | ===3D Structures in Different Organisms=== | ||
''Mycobacterium tuberculosis'' | ''Mycobacterium tuberculosis'' | ||
| Line 78: | Line 77: | ||
===Secondary Structural Elements=== | ===Secondary Structural Elements=== | ||
[[Image:Secondary2iyq.PNG|300px|left|thumb| Secondary Structural Elements<ref name="PDB" />]] | [[Image:Secondary2iyq.PNG|300px|left|thumb| Secondary Structural Elements<ref name="PDB" />]] | ||
| - | Using [[2iyq]] as an example, the secondary structure of shikimate kinase can be observed in terms of strands and helices shown <scene name='56/564039/Helixsheet/1'>here</scene>. There are 5 strands, shown in yellow, and 9 helices (8 α-helices and | + | Using [[2iyq]] as an example, the secondary structure of shikimate kinase can be observed in terms of strands and helices shown <scene name='56/564039/Helixsheet/1'>here</scene>. There are 5 strands, shown in yellow, and 9 helices (8 α-helices and a single 3<sub>10</sub> helix) which are shown in pink. Shown to the left is a 2D depiction of the secondary structural content of shikimate kinase (strands are yellow, helices are pink) and corresponding residues. |
| Line 90: | Line 89: | ||
===Isoforms=== | ===Isoforms=== | ||
| - | In '' Escherichia coli'' the shikimate kinase reaction is catalyzed by two different isoforms: SKI and SKII (Km= 20 μM Km= 200 μM, respectively) with 30% sequence identify. This is very unusual for two isoforms to occur in the middle of a biosynthetic pathway. Therefore, what may be occurring is that shikimate is at a branch point for two pathways. It is believed that SKII is the isoform participating in chorismate biosynthesis, while the role of the SKI isoform is unclear.<ref name=" | + | In '' Escherichia coli'' the shikimate kinase reaction is catalyzed by two different isoforms: SKI and SKII (Km= 20 μM Km= 200 μM, respectively) with 30% sequence identify. This is very unusual for two isoforms to occur in the middle of a biosynthetic pathway. Therefore, what may be occurring is that shikimate is at a branch point for two pathways. It is believed that SKII is the isoform participating in chorismate biosynthesis, while the role of the SKI isoform is unclear.<ref name="3dstructure">The three-dimensional structure of shikimate kinase. Journal of Molecular Biology http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S0022283698917557</ref> |
| + | |||
===Oligomeric State=== | ===Oligomeric State=== | ||
| - | + | Through experiments involving size exclusion liquid chromatography and gel filtration chromatography, the oligomeric state of homogeneous MtSK was found. The molecular mass was found to be 20.7 kDa and when compared to the suggested value of 18.5 kDa, it was found that MtSK is a monomer in solution.<ref>The Mode of Action of Recombinant Mycobacterium tuberculosis Shikimate Kinase: Kinetics and Thermodynamics Analyses http://www.plosone.org/article/info:doi/10.1371/journal.pone.0061918</ref> | |
| - | + | ||
| - | + | ||
| - | Through experiments involving size exclusion liquid chromatography and gel filtration chromatography, the oligomeric state of homogeneous MtSK was found. The molecular mass was found to be 20.7 kDa and when compared to the suggested value of 18.5 kDa, it was found that MtSK is a monomer in solution.<ref>The Mode of Action of Recombinant Mycobacterium tuberculosis Shikimate Kinase: Kinetics and Thermodynamics Analyses http://www.plosone.org/article/info:doi/10.1371/journal.pone.0061918</ref> | + | |
===Active Residues=== | ===Active Residues=== | ||
| Line 110: | Line 107: | ||
===Ligands=== | ===Ligands=== | ||
The liganded state of SK includes binary complexes with SO4 or MgADP ([[1l4y]]) and ternary complexes with shikimate as the first ligand and SO4 ([[2g1k]]), ADP ([[1u8a]]), MgADP, or AMPPCP (an ATP analogue) ([[1zyu]]) as the second ligand.<ref name="miscinfo" /> | The liganded state of SK includes binary complexes with SO4 or MgADP ([[1l4y]]) and ternary complexes with shikimate as the first ligand and SO4 ([[2g1k]]), ADP ([[1u8a]]), MgADP, or AMPPCP (an ATP analogue) ([[1zyu]]) as the second ligand.<ref name="miscinfo" /> | ||
| - | |||
| - | |||
| - | ADP binds in P-loop, Mg+2 binds nearby and is essential for enzyme activity. Shikimate binding occurs with helices α2, α3, and α4 (N-terminal region).<ref name="skstructure"> | ||
| - | |||
Magnesium ion hold influence over the position of shikimate hydroxy groups. This ion has a role in the transfer of the γ-phosphate of ATP to the 3-hydroxy group on shikimate. Chloride ions increase enzyme affinity for ADP and ATP and help to bind the nucleotide substrate in correct orientation.<ref name="mgcl"> Effects of the magnesium and chloride ions and shikimate on the structure of shikimate kinase from Mycobacterium tuberculosis Structural Biology and Crystallization Communications | Magnesium ion hold influence over the position of shikimate hydroxy groups. This ion has a role in the transfer of the γ-phosphate of ATP to the 3-hydroxy group on shikimate. Chloride ions increase enzyme affinity for ADP and ATP and help to bind the nucleotide substrate in correct orientation.<ref name="mgcl"> Effects of the magnesium and chloride ions and shikimate on the structure of shikimate kinase from Mycobacterium tuberculosis Structural Biology and Crystallization Communications | ||
| Line 140: | Line 133: | ||
MgADP | MgADP | ||
| - | Shikimate interacts | + | Shikimate interacts viah intermolecular hydrogen bonds with Asp34, Arg58, Gly80, and Arg136<ref name="miscinfo" /><ref name="inhibitors"> Identification of new potential Mycobacterium tuberculosis shikimate kinase inhibitors through molecular docking simulations. Journal of Molecular Modeling http://link.springer.com.prox.lib.ncsu.edu/article/10.1007%2Fs00894-011-1113-5 |
</ref> | </ref> | ||
| Line 159: | Line 152: | ||
===Protein Fold=== | ===Protein Fold=== | ||
| - | Protein folding from a random coil to native state with correct 3D structure is essential for proper protein function. The primary sequence of amino acids determines the folded structure. The topology of this enzyme | + | Protein folding from a random coil to native state with correct 3D structure is essential for proper protein function. The primary sequence of amino acids determines the folded structure. The topology of this enzyme shows an alpha/beta/alpha fold consisting of a 5-stranded central parallel beta sheet and 8 surrounding alpha-helices.<ref name="miscinfo" /> |
| - | Shikimate kinase is a convenient protein to use in protein folding studies. This is because it is one of the smallest kinases and is a monomeric enzyme. It has been shown that the proposed refolding model includes a rapid hydrophobic collapse and then a slower secondary structure formation.<ref name="fold">The refolding of type II shikimate kinase from Erwinia chrysanthemi | + | Shikimate kinase is a convenient protein to use in protein folding studies. This is because it is one of the smallest kinases and is a monomeric enzyme lacking disulfide bonds. It has been shown that the proposed refolding model includes a rapid hydrophobic collapse and then a slower secondary structure formation.<ref name="fold">The refolding of type II shikimate kinase from Erwinia chrysanthemi |
after denaturation in urea. European Journal of Biochemistry http://onlinelibrary.wiley.com/doi/10.1046/j.1432-1033.2002.ejb.02862.x/pdf</ref> | after denaturation in urea. European Journal of Biochemistry http://onlinelibrary.wiley.com/doi/10.1046/j.1432-1033.2002.ejb.02862.x/pdf</ref> | ||
| - | + | Look into how the protein fold could be disrupted (ie pH, temperature studies etc) | |
| Line 184: | Line 177: | ||
Circular Dichroism | Circular Dichroism | ||
| - | Spectroscopy | ||
| - | Heavy atom derivatisation | ||
| - | Electron density mapping | ||
| - | |||
| - | Chromatography | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
[[Image:Shikimate_kinase_reaction.png|400px|left|thumb| Shikimate kinase catalyzed reaction]] | [[Image:Shikimate_kinase_reaction.png|400px|left|thumb| Shikimate kinase catalyzed reaction]] | ||
| - | |||
===Reaction Pathway=== | ===Reaction Pathway=== | ||
Revision as of 04:05, 5 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Contents |
Shikimate Kinase
|
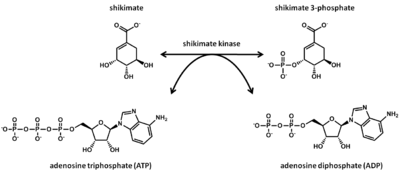
Shikimate kinase (SK) is an enzyme which participates in the fifth step of the shikimate pathway. The functional role shikimate kinase plays in this pathway is to catalyze the ATP-dependent phosphorylation of shikimate into shikimate 3-phosphate (3-phosphoshikimate). This aids in the synthesis of chorismate, which is the precursor to aromatic amino acids and secondary metabolites. Shikimate kinase belongs to the nucleoside monophosphate (NMP) kinase family, which phosphorylates a protein leading to a functional change in the phosphorylated protein. SK is of the transferase class, which acts to transfer a functional group from the donor to acceptor molecule. The protein fold consists of 8 α-helices and 5 β-strands. The length of shikimate kinase is 173 residues and it is found in the cytoplasm. This is a subclass of α/β proteins, meaning an α/β domain exists. SK is composed of Molecule A and B, which together form the asymmetric unit, with SK functioning as a monomer. SK consists of the CORE, LID, and substrate-binding domains. ATP is the co-substrate while magnesium ion is the co-factor. The shikimate pathway is present in bacteria, fungi, higher plants, algae, and apicomplexan. This enzyme has been experimentally observed predominantly in the organism Mycobacterium tuberculosis, but also in Helicobacter pylori, Bacteroides thetaiotaomicron, Campylobacter jejuni, Aquifex aeolicus, Coxiella burnetii, Arabidopsis thaliana. The aroK gene encodes for SK in Mycobacterium tuberculosis. This enzyme is a protein target for rational drug design, which holds great potential due to shikimate kinase being absent in mammals.
Structure
The crystal structure of 2iyq from the Protein Data Bank [1] showing shikimate kinase from Mycobacterium tuberculosis complexed with ADP and shikimate is shown to the right as the . In addition, the 2D model of 2gij, showing the space filling details of the asymmetric unit of MtSK, is shown to the left.
3D Structures in Different Organisms
Mycobacterium tuberculosis
2iyt - MtSK (unliganded, open lid)
1iyw - MtSK (open lid) + ATP
2g1k, 2iyr, 2iyx - MtSK + shikimate
2iys - MtSK (open lid) + shikimate
2iyy - MtSK + shikimate-3-phosphate
2iys - MtSK (open lid) + shikimate
2iyu, 2iyv - MtSK (open lid) + ADP
1l4u, 1l4y, 1u8a, 2dft - MtSK + ADP
2iyw - MtSK (open lid) + ATP
3baf - MtSK + AMP-PNP
2dfn, 1we2, 2iyq, 1u8a - MtSK + ADP + shikimate
2iyz - MtSK + ADP + shikimate-3-phosphate
4bqs - MtSK + ADP + shikimic acid
2g1j - MtSK + AMPPCP (ATP analogue)+ shikimic acid
1zyu - MtSK + AMPPCP + shikimate
Helicobacter pylori
1zuh - HpSK
3mrs - HpSK (mutant)
1zui - HpSK + shikimate
3n2e - HpSK (mutant) + inhibitor
3muf - HpSK + ADP + shikimate-3-phosphate
Other Organisms
2pt5 - SK - Aquifex Aeolicus
3trf - SK - Coxiella burnetii
3vaa - SK - Bacteroides thetaiotaomicron
1via - SK - Campylobacter jejuni
3nwj - SK - Arabidopsis thaliana
Secondary Structural Elements
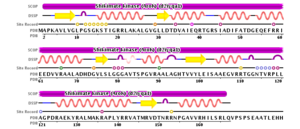
Using 2iyq as an example, the secondary structure of shikimate kinase can be observed in terms of strands and helices shown . There are 5 strands, shown in yellow, and 9 helices (8 α-helices and a single 310 helix) which are shown in pink. Shown to the left is a 2D depiction of the secondary structural content of shikimate kinase (strands are yellow, helices are pink) and corresponding residues.
Isoforms
In Escherichia coli the shikimate kinase reaction is catalyzed by two different isoforms: SKI and SKII (Km= 20 μM Km= 200 μM, respectively) with 30% sequence identify. This is very unusual for two isoforms to occur in the middle of a biosynthetic pathway. Therefore, what may be occurring is that shikimate is at a branch point for two pathways. It is believed that SKII is the isoform participating in chorismate biosynthesis, while the role of the SKI isoform is unclear.[2]
Oligomeric State
Through experiments involving size exclusion liquid chromatography and gel filtration chromatography, the oligomeric state of homogeneous MtSK was found. The molecular mass was found to be 20.7 kDa and when compared to the suggested value of 18.5 kDa, it was found that MtSK is a monomer in solution.[3]
Active Residues
Using 2iyq as an example, the active residues were binding in shikimate kinase occurs is shown to the left. There are three domains present in Shikimate kinase: the CORE domain, and substrate-binding (SB) domain, and LID domain. Each domain plays a role in binding and is related to the active residues. The binding site for nucleotides is in the CORE, shikimate binds in the substrate-binding domain, and once ATP or shikimate binds, the LID domain closes over the active site.[4]
The CORE domain spans from residues 9-17 (phosphate binding loop), 148-155 (AB-loop), and 101-110 (segment which includes alpha6 from 104-110). The SB domain is comprised of residues 32-93 and consists of a sub-domain from residues 32-61 which relates to the NMP-binding domain in NMP kinases. Finally, the LID domain consists of residues 112-124. A global motion leads to LID flapping over the active site upon binding the first substrate (whether shikimate or nucleotide). This causes a change from the open to closed conformation. [5]
Ligands
The liganded state of SK includes binary complexes with SO4 or MgADP (1l4y) and ternary complexes with shikimate as the first ligand and SO4 (2g1k), ADP (1u8a), MgADP, or AMPPCP (an ATP analogue) (1zyu) as the second ligand.[5]
Magnesium ion hold influence over the position of shikimate hydroxy groups. This ion has a role in the transfer of the γ-phosphate of ATP to the 3-hydroxy group on shikimate. Chloride ions increase enzyme affinity for ADP and ATP and help to bind the nucleotide substrate in correct orientation.[6] Magnesium ion binds in the active site.
SO4
SO4 interacts with Arg117 and distorts the nucleotide binding site[5]
ADP interacts with P-loop, AB-loop, and alpha6[5] MgADP
Shikimate interacts viah intermolecular hydrogen bonds with Asp34, Arg58, Gly80, and Arg136[5][7]
AMPPCP does not adequately show ATP and SK interaction due to loss of beta-phosphate and P-loop nitrogen atoms bonding.
Ligands associated with shikimate kinase include ADP, CL, shikimate (SKM), and TRS.
Adenosine-5'-disphosphate (
Chloride ion ()
(3R,4S,5R)-3,4,5-trihydroxycyclohex-1-ene-1-caroxylic acid ()
2-amino-2-hydroxymethyl-propane-1,3-diol (TRS)
Protein Fold
Protein folding from a random coil to native state with correct 3D structure is essential for proper protein function. The primary sequence of amino acids determines the folded structure. The topology of this enzyme shows an alpha/beta/alpha fold consisting of a 5-stranded central parallel beta sheet and 8 surrounding alpha-helices.[5]
Shikimate kinase is a convenient protein to use in protein folding studies. This is because it is one of the smallest kinases and is a monomeric enzyme lacking disulfide bonds. It has been shown that the proposed refolding model includes a rapid hydrophobic collapse and then a slower secondary structure formation.[8]
Look into how the protein fold could be disrupted (ie pH, temperature studies etc)
Methods Used to Solve
The following methods have been used in solving for the structure of SK:
Crystallization via hanging-drop vapor-diffusion
Molecular replacement methods for structure refinement
multiple isomorphous replacement
Fourier calculations
Electrospray mass spectrometry
Dynamic light scattering
Circular Dichroism
Mechanism of Action
Reaction Pathway
SK is found in the shikimate pathway, which is present in bacteria, fungi, higher plants, algae, and apicomplexan. This pathway serves to convert erythrose-4-phosphate into chorismate. Chorismate is a required intermediate in the biosynthesis of aromatic amino acids and secondary metabolites. Shikimate kinase is the catalyst for the fifth step of the shikimate pathway, where shikimate (SKM) is converted into shikimate-3-phosphate (S3P) and ATP serves as a co-substrate. It has been found experimentally that SK and shikimate binding involves two steps: substrate attaches to binding site in the first step and LID closure occurs in the second step.[5]
Reaction Mechanism
Inhibitors
When sulfate binds as a ligand, it distorts the nucleotide binding site. This leads to a large decrease of motion in comparison to a nucleotide binding. The implication of this is that Ser16OG and Asp32OD2 are no longer close enough to hydrogen bond and bring about contact between the CORE and SB domains.[5] This may not strictly fall under the definition of an inhibitor, but is still worth noting.
Describe how protein functions. Structures of Ligands/inhibitors/important steps in reaction pathway.
Implications or Possible Application
Enzymes present in the shikimate pathway are important in microorganism survival yet absent in mammals. This means the enzymes of the SKM pathway are good candidates for antimicrobial agents, herbicides, inhibitors, and anti-parasitic drug target. [5][7]
Studies of potential inhibitors of SK have been conducted. This is important in the identification of potential drugs against tuberculosis (TB), a curable infectious disease found mainly in developing countries. TB is curable, yet one hundred million people are infected annually with approximately three million resulting deaths. TB resurges as a health problem due to multi-drug resistant, extensively drug resistant, and totally-drug resistant strains of Mt.[7]
One way potential MtSK inhibitors have been studied is through molecular docking simulations experiments. These experiments predict the conformation of a receptor-ligand complex to analyze all possible positions of the ligand to make a selection for the best position. Virtual screening was also used to identify active molecules compared to a specific protein target. These studies have been able to confirm staurosporine as a known SK inhibitor.[7]
Antimicrobial Agents
Herbicides
Inhibitors
Anti-parasitic Drug Target
Medical Importance
Medical importance - related disease, if used as drug target.
Other uses (ie antibiotics etc)
References
- ↑ 1.0 1.1 1.2 Protein Data Bank http://www.rcsb.org/pdb/explore.do?structureId=2IYQ
- ↑ The three-dimensional structure of shikimate kinase. Journal of Molecular Biology http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S0022283698917557
- ↑ The Mode of Action of Recombinant Mycobacterium tuberculosis Shikimate Kinase: Kinetics and Thermodynamics Analyses http://www.plosone.org/article/info:doi/10.1371/journal.pone.0061918
- ↑ UniProt http://www.uniprot.org/uniprot/P0A4Z2
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Mechanism of Phosphoryl Transfer Catalyzed by Shikimate Kinase from Mycobacterium tuberculosis. Journal of Molecular Biology http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S0022283606011685#
- ↑ Effects of the magnesium and chloride ions and shikimate on the structure of shikimate kinase from Mycobacterium tuberculosis Structural Biology and Crystallization Communications http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2330112/
- ↑ 7.0 7.1 7.2 7.3 Identification of new potential Mycobacterium tuberculosis shikimate kinase inhibitors through molecular docking simulations. Journal of Molecular Modeling http://link.springer.com.prox.lib.ncsu.edu/article/10.1007%2Fs00894-011-1113-5
- ↑ The refolding of type II shikimate kinase from Erwinia chrysanthemi after denaturation in urea. European Journal of Biochemistry http://onlinelibrary.wiley.com/doi/10.1046/j.1432-1033.2002.ejb.02862.x/pdf