We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 777
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
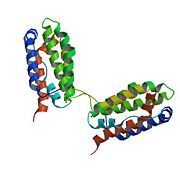
| - | <Structure load='2H24' size='300' frame='true' align='right' caption='The interleukin 10 monomer. PDB 2H24' scene='Insert optional scene name here' /> | + | <Structure load='2H24' size='300' frame='true' align='right' caption='The interleukin 10 monomer. PDB 2H24<ref>RCSB Protein Data Bank, www.rcsb.org</ref>' scene='Insert optional scene name here' /> |
Interleukin 10 (IL-10) belongs to a class of proteins called cytokines. Cytokines are chemical messengers that are produced by and act on cells of the immune system.<ref>National Institute of Allergy and Infectious Diseases, ”Immune Cells and their Products: Cytokines”. Web. 16Nov2013 <http://www.niaid.nih.gov/topics/immuneSystem/immuneCells/Pages/cytokines.aspx></ref> It is also part of the IL-10 family of cytokines, which also includes IL-19, IL-20, IL-22, IL-24 and IL-26. This grouping was made based on similarities in structure, function, and location of the encoding genes.<ref name="sabat">Sabat, Robert. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010; 21:315–324</ref> IL-10 has also previously been called cytokine synthesis inhibitory factor (CSIF) because of its anti-inflammatory properties and ability to inhibit the production of other cytokines.<ref name="dewaal">de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries, JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991 Nov; 174(5):1209-1220</ref> The ability to limit the immune response to pathogens is crucial to preventing self-inflicted damage to the host. This cytokine is expressed in many eukaryotic hosts, as well as some viruses. A quick protein BLAST of the human IL-10 sequence reveals homology with IL-10 of many other primate species, as well as that found in bats, horses, elephants, rodents, sheep, cats and birds. The remainder of this page will focus on human IL-10. | Interleukin 10 (IL-10) belongs to a class of proteins called cytokines. Cytokines are chemical messengers that are produced by and act on cells of the immune system.<ref>National Institute of Allergy and Infectious Diseases, ”Immune Cells and their Products: Cytokines”. Web. 16Nov2013 <http://www.niaid.nih.gov/topics/immuneSystem/immuneCells/Pages/cytokines.aspx></ref> It is also part of the IL-10 family of cytokines, which also includes IL-19, IL-20, IL-22, IL-24 and IL-26. This grouping was made based on similarities in structure, function, and location of the encoding genes.<ref name="sabat">Sabat, Robert. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010; 21:315–324</ref> IL-10 has also previously been called cytokine synthesis inhibitory factor (CSIF) because of its anti-inflammatory properties and ability to inhibit the production of other cytokines.<ref name="dewaal">de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries, JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991 Nov; 174(5):1209-1220</ref> The ability to limit the immune response to pathogens is crucial to preventing self-inflicted damage to the host. This cytokine is expressed in many eukaryotic hosts, as well as some viruses. A quick protein BLAST of the human IL-10 sequence reveals homology with IL-10 of many other primate species, as well as that found in bats, horses, elephants, rodents, sheep, cats and birds. The remainder of this page will focus on human IL-10. | ||
== Structure == | == Structure == | ||
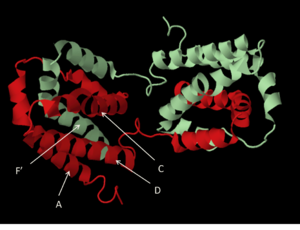
| - | [[Image:4helixbundle.png|300px|left|thumb|The four-helix bundle. Image built in PyMOL<ref>www.pymol.org</ref>using PDB 2H24<ref>RCSB Protein Data Bank, www.rcsb.org</ref>]] | + | [[Image:4helixbundle.png|300px|left|thumb|The four-helix bundle. Image built in PyMOL<ref>http://www.pymol.org</ref> using PDB 2H24<ref>RCSB Protein Data Bank, http://www.rcsb.org</ref>]] |
The cytokine structure is an intercalated dimer of two identical polypeptide chains, each 178 amino acids in length.<ref name="moore">Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the Interleukin-10 receptor. Annu. Rev. Immunol. 2001.19:683-765</ref> The monomers are made up of six amphipathic helices, with two helices of one monomer interacting with four of the other monomer, creating two distinct domains.A four-helix bundle is made up of helices A, C, D and F’ (and A’, C’ D’ and F). This feature is a signature element of all helical cytokines.<ref>Zdanov, A. Structural analysis of cytokines comprising the IL-10 family. Cytokine Growth Factor Rev. 2010; 21:325-330</ref> The structure is stabilized by <scene name='56/564053/Il-10_disulfide_bonds/2'>two disulfide bridges</scene> formed between the first and third cysteine residues and the second and fourth cysteine residues (Cys12 with Cys108, and Cys62 with Cys114). In the image, the disulfide bridges are shown in yellow. Reduction of the disulfide bonds in recombinant IL-10 has been shown to reduce helical content measured by circular dichroism and results in a lack of in vitro biological activity.<ref>Windsor WT, Syto R, Tsarbopoulos A, et al. Disulfide bond assignments and secondary structure analysis of human and murine interleukin 10. Biochemistry. 1993; 32:8807-8815</ref>[[Image:1ilk bio r 500.jpg |right|thumb| another view of the biologically active homodimer]] | The cytokine structure is an intercalated dimer of two identical polypeptide chains, each 178 amino acids in length.<ref name="moore">Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the Interleukin-10 receptor. Annu. Rev. Immunol. 2001.19:683-765</ref> The monomers are made up of six amphipathic helices, with two helices of one monomer interacting with four of the other monomer, creating two distinct domains.A four-helix bundle is made up of helices A, C, D and F’ (and A’, C’ D’ and F). This feature is a signature element of all helical cytokines.<ref>Zdanov, A. Structural analysis of cytokines comprising the IL-10 family. Cytokine Growth Factor Rev. 2010; 21:325-330</ref> The structure is stabilized by <scene name='56/564053/Il-10_disulfide_bonds/2'>two disulfide bridges</scene> formed between the first and third cysteine residues and the second and fourth cysteine residues (Cys12 with Cys108, and Cys62 with Cys114). In the image, the disulfide bridges are shown in yellow. Reduction of the disulfide bonds in recombinant IL-10 has been shown to reduce helical content measured by circular dichroism and results in a lack of in vitro biological activity.<ref>Windsor WT, Syto R, Tsarbopoulos A, et al. Disulfide bond assignments and secondary structure analysis of human and murine interleukin 10. Biochemistry. 1993; 32:8807-8815</ref>[[Image:1ilk bio r 500.jpg |right|thumb| another view of the biologically active homodimer]] | ||
Revision as of 18:46, 6 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Introduction
| |||||||||||