Sandbox Reserved 766
From Proteopedia
| Line 13: | Line 13: | ||
== Structure == | == Structure == | ||
'''Asparagine Synthetase''' is a homodimer comprised of two domains ligated together. ASPS consists of a | '''Asparagine Synthetase''' is a homodimer comprised of two domains ligated together. ASPS consists of a | ||
| - | <scene name='56/564042/Glutamine_amidotransferase/1'>Glutamine Amidotransferase Type-2</scene> (residues 2-191) and and <scene name='56/564042/Asparagine_synthetase_complex/1'>Asparagine Synthetase</scene> complex (residues 213-536). There are two specific domains that contain the substrate binding sites. The N-terminal domain which contains two layers of anti-parallel beta sheets comprised of six layers each, this is where the Glutamine binds. A C-terminal domain also exists, it is comprised of five parallel beta sheets with alpha helices on either side of it, <ref>"RCSB Protein Data Bank - RCSB PDB - 1CT9 Structure Summary." RCSB Protein Data Bank - RCSB PDB - 1CT9 Structure Summary. N.p., n.d. Web. 01 Dec. 2013.</ref> this is where the Mg2+, ATP and Aspartic Acid bind. The two active sites contained within the terminal domains are linked together by a tunnel of hydrophobic and polar surface amino acid residues. Specifically <scene name='56/564042/Residue_365/1'>Residue 365</scene> is important for the binding of the Beta-Asparty-AMP intermediate into the enzyme. | + | <scene name='56/564042/Glutamine_amidotransferase/1'>Glutamine Amidotransferase Type-2</scene> (residues 2-191) and and <scene name='56/564042/Asparagine_synthetase_complex/1'>Asparagine Synthetase</scene> complex (residues 213-536). There are two specific domains that contain the substrate binding sites. The N-terminal domain which contains two layers of anti-parallel beta sheets comprised of six layers each, this is where the Glutamine binds. A C-terminal domain also exists, it is comprised of five parallel beta sheets with alpha helices on either side of it, <ref>"RCSB Protein Data Bank - RCSB PDB - 1CT9 Structure Summary." RCSB Protein Data Bank - RCSB PDB - 1CT9 Structure Summary. N.p., n.d. Web. 01 Dec. 2013.</ref> this is where the Mg2+, ATP and Aspartic Acid bind. The two active sites contained within the terminal domains are linked together by a tunnel of hydrophobic and polar surface amino acid residues. Specifically <scene name='56/564042/Residue_365/1'>Residue 365</scene> is important for the binding of the Beta-Asparty-AMP intermediate into the enzyme. |
| - | + | ||
===Amino Acid Composition=== | ===Amino Acid Composition=== | ||
| Line 34: | Line 33: | ||
===Ovarian Cancer Predictive Bio-Marker=== | ===Ovarian Cancer Predictive Bio-Marker=== | ||
| - | Recent studies have been trying to find a potential link between the amount of ASNS activity in certain cell cultures as a precursor for L-Asparaginase activity. L-Asparaginase is an enzyme that degrades Asparagine and has been used since the 1970's in basic cancer treatment plans for acute lymphoblastic leukemia (site MCT). The body will naturally secrete L-Asparaginase as a response to a potential forming tumor or potential cancer cell aggregation. These studies are finding a correlation between the amount of ASNS being expressed prior to the expression of L-Asparaginase synthetase, since L-Asparaginase has only been shown to help combat lymphoblastic leukemia this would not be a direct treatment but rather an indicator that something is happening that requires attention and hopefully allow doctors to screen for ASNS activity and catch ovarian cancer in it's earlier stages when it is more treatable and easier to remove from the body. | + | Recent studies have been trying to find a potential link between the amount of ASNS activity in certain cell cultures as a precursor for L-Asparaginase activity. L-Asparaginase is an enzyme that degrades Asparagine and has been used since the 1970's in basic cancer treatment plans for acute lymphoblastic leukemia (site MCT). The body will naturally secrete L-Asparaginase as a response to a potential forming tumor or potential cancer cell aggregation. These studies are finding a correlation between the amount of ASNS being expressed prior to the expression of L-Asparaginase synthetase,<ref>"Asparagine Synthetase as a Causal, Predictive Biomarker for L-asparaginase Activity in Ovarian Cancer Cells." Asparagine Synthetase as a Causal, Predictive Biomarker for L-asparaginase Activity in Ovarian Cancer Cells. N.p., n.d. Web. 02 Dec. 2013.<ref/> since L-Asparaginase has only been shown to help combat lymphoblastic leukemia this would not be a direct treatment but rather an indicator that something is happening that requires attention and hopefully allow doctors to screen for ASNS activity and catch ovarian cancer in it's earlier stages when it is more treatable and easier to remove from the body. |
==References== | ==References== | ||
Revision as of 04:09, 7 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
Asparagine Synthetase (ASNS) is an enzyme that catalyzes the conversion of Aspartic Acid to Asparagine through an ATP dependent amination reaction, using Mg2+ as a co-factor. ASNS can be found in both plants and mammals, however, in plants there are two forms ASNS-A and ASNS-B[1]. Both forms use the same starting molecule of Aspartic Acid and produce Asparagine, the only difference coming from the source of the Nitrogen. ASNS-A uses inorganic Nitrogen in the form of ammonia or diatomic Nitrogen and ASNS-B uses Glutamine as its Nitrogen source; ASNS-B will be the focus of this page because it is very similar and undergoes the same reaction as ASNS in mammals. ASNS is in almost every somatic mammalian cell but is expressed in exponentially higher concentrations in the pancreas.
Structure
Asparagine Synthetase is a homodimer comprised of two domains ligated together. ASPS consists of a (residues 2-191) and and complex (residues 213-536). There are two specific domains that contain the substrate binding sites. The N-terminal domain which contains two layers of anti-parallel beta sheets comprised of six layers each, this is where the Glutamine binds. A C-terminal domain also exists, it is comprised of five parallel beta sheets with alpha helices on either side of it, [2] this is where the Mg2+, ATP and Aspartic Acid bind. The two active sites contained within the terminal domains are linked together by a tunnel of hydrophobic and polar surface amino acid residues. Specifically is important for the binding of the Beta-Asparty-AMP intermediate into the enzyme.
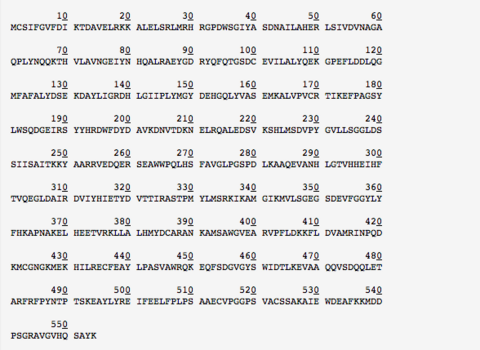
Amino Acid Composition
The Entire amino acid sequence for this enzyme complex can be found below. ASNS consists 1662 residues or 554 amino acids with a molecular weight of 62.7 kDa.
Function
Asparagine Synthetase catalyzes the interconversion of Aspartic Acid and Glutamine to Asparagine and Glutamic Acid. This is not a straight forward transferase reaction since the interconversion is not directly between Aspartic Acid and Glutamine. Instead this reaction more closely resembles a two part ATP dependent ligase reaction. ASNS has two distinct pockets; one where Adenosine Triphosphate (ATP) binds and is stabilized by the hydrogen bonds it forms with Ser 346 (Gamma Oxygen) and amide groups of Val 272, Leu 232 and Gly 347. This binding of ATP to the N-Terminal domain is to stabilize the intermediate it forms with the Aspartic Acid; Beta-Aspartyl AMP (BAspAMP). This intermediate complex has to bind to the ASNS enzyme before the Glutamine binds into its pocket to establish coordination of the binding sites within the ASNS enzyme. After the BAspAMP intermediate binds in its pocket and establishes the coordination of the enzyme the Glutamine binds to its pocket in the C-Terminal domain and is stabilized specifically by its bonds with Arg 49, Asn 74, Glu 76, and Asp 98 residues.[3]