We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
m |
|||
| Line 21: | Line 21: | ||
Usually these domains are involved in redox phenomena, which lead to disulfide bounds creation. Here these domains are inactive but play an important role in the polymerization of CASQ2. | Usually these domains are involved in redox phenomena, which lead to disulfide bounds creation. Here these domains are inactive but play an important role in the polymerization of CASQ2. | ||
| - | === Polymer Structure === <ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | + | === Polymer Structure === <ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> |
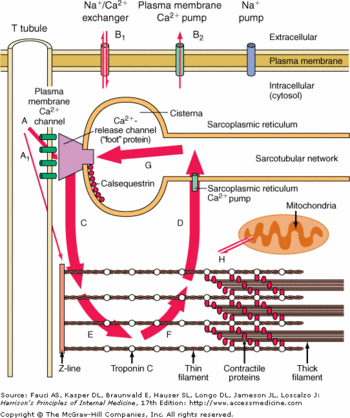
Inside the sarcoplasmic reticulum lumen, CASQ2 polymerizes to form <scene name='56/568018/Dimer/1'>homodimers</scene>, homotetramers and homooligomers. | Inside the sarcoplasmic reticulum lumen, CASQ2 polymerizes to form <scene name='56/568018/Dimer/1'>homodimers</scene>, homotetramers and homooligomers. | ||
There are two types of dimerisation: the front-to-front form and the back-to-back form. | There are two types of dimerisation: the front-to-front form and the back-to-back form. | ||
| Line 44: | Line 44: | ||
== Interaction between CASQ2 and <!-- (plutôt Triadin et Junctin) -->RYR == | == Interaction between CASQ2 and <!-- (plutôt Triadin et Junctin) -->RYR == | ||
| - | === Binding sites === <ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> | + | === Binding sites === <ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> |
CASQ2 is anchored into the membrane of SR thanks to two integral proteins: the triadin and the junctin. Triadin as well as Juctin can bind to CASQ2 because of its KEKE motif between the amino acids 210 and 224 for the triadin. The binding site of CASQ2 for the both protein is the Asp-rich region of the C-terminal region. | CASQ2 is anchored into the membrane of SR thanks to two integral proteins: the triadin and the junctin. Triadin as well as Juctin can bind to CASQ2 because of its KEKE motif between the amino acids 210 and 224 for the triadin. The binding site of CASQ2 for the both protein is the Asp-rich region of the C-terminal region. | ||
Triadin and Junctin interact with Ryanodin Receptor (RyR). | Triadin and Junctin interact with Ryanodin Receptor (RyR). | ||
| Line 56: | Line 56: | ||
<!-- Source: Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15731387 --> | <!-- Source: Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15731387 --> | ||
| - | == Regulation of CASQ2 == <ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> | + | == Regulation of CASQ2 == <ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> |
CASQ2 can be phosphorylated by three different kinases: casein kinase I (CK I), casein kianse II (CK II) and ε protein kinase C1 (εPKC1). CK II is located in the SR and is able to phosphorylate Ser 378, Ser 382 and Ser 386. These residues are on the C-terminal domain. The consensus sequence recognized by CK II is Ser/Thr-X-X-Asp/Glu. More there are acidic residues after this consensus sequence, more the probabilty of phosphorylation increases. | CASQ2 can be phosphorylated by three different kinases: casein kinase I (CK I), casein kianse II (CK II) and ε protein kinase C1 (εPKC1). CK II is located in the SR and is able to phosphorylate Ser 378, Ser 382 and Ser 386. These residues are on the C-terminal domain. The consensus sequence recognized by CK II is Ser/Thr-X-X-Asp/Glu. More there are acidic residues after this consensus sequence, more the probabilty of phosphorylation increases. | ||
Revision as of 16:21, 2 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 4.0 4.1 4.2 http://www.ncbi.nlm.nih.gov/pubmed/15050380
- ↑ 5.0 5.1 http://www.ncbi.nlm.nih.gov/pubmed/15731387