This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
m |
|||
| Line 56: | Line 56: | ||
<!-- Source: Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15731387 --> | <!-- Source: Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005) Lien: http://www.ncbi.nlm.nih.gov/pubmed/15731387 --> | ||
| - | == Regulation of CASQ2 == | + | == Regulation of CASQ2 == |
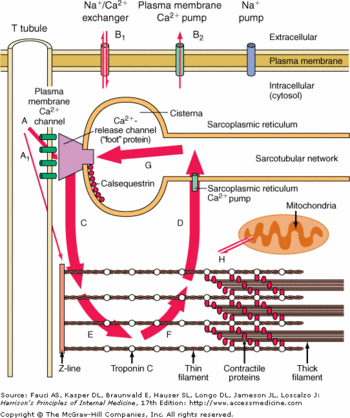
CASQ2 can be phosphorylated by three different kinases: casein kinase I (CK I), casein kianse II (CK II) and ε protein kinase C1 (εPKC1).<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> CK II is located in the SR and is able to phosphorylate Ser 378, Ser 382 and Ser 386. These residues are on the C-terminal domain.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> The consensus sequence recognized by CK II is Ser/Thr-X-X-Asp/Glu. <ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref>More there are acidic residues after this consensus sequence, more the probabilty of phosphorylation increases.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> | CASQ2 can be phosphorylated by three different kinases: casein kinase I (CK I), casein kianse II (CK II) and ε protein kinase C1 (εPKC1).<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> CK II is located in the SR and is able to phosphorylate Ser 378, Ser 382 and Ser 386. These residues are on the C-terminal domain.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> The consensus sequence recognized by CK II is Ser/Thr-X-X-Asp/Glu. <ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref>More there are acidic residues after this consensus sequence, more the probabilty of phosphorylation increases.<ref name="Calsequestrin and the calcium release channel of skeletal and cardiac muscle (Beard et Al., 2004)">http://www.ncbi.nlm.nih.gov/pubmed/15050380</ref> | ||
Revision as of 16:27, 2 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 http://www.ncbi.nlm.nih.gov/pubmed/15050380
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 http://www.ncbi.nlm.nih.gov/pubmed/15731387