Sandbox Reserved 816
From Proteopedia
| Line 2: | Line 2: | ||
{{Sandbox_Reserved_ESBS}} | {{Sandbox_Reserved_ESBS}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| + | |||
| + | <Structure load='4l3a' size='500' frame='true' align='right' caption='Internalin K bound to MVP' scene='Insert optional scene name here' /> | ||
| + | |||
| Line 11: | Line 14: | ||
'''Internalin K''' is involved in [http://en.wikipedia.org/wiki/Listeria_monocytogenes Listeria monocytogene] ability to escape from autophagy by recruitment of [http://en.wikipedia.org/wiki/Major_vault_protein major vault protein] to the bacterial surface. | '''Internalin K''' is involved in [http://en.wikipedia.org/wiki/Listeria_monocytogenes Listeria monocytogene] ability to escape from autophagy by recruitment of [http://en.wikipedia.org/wiki/Major_vault_protein major vault protein] to the bacterial surface. | ||
| - | |||
| Line 19: | Line 21: | ||
===='''Internalin family's generalities'''==== | ===='''Internalin family's generalities'''==== | ||
| + | [[Image:Internalin.jpg]] | ||
| + | |||
| + | Internalin family | ||
| + | |||
| + | ---- | ||
'''L.monocytogenes''' uses a lot of virulence factors to initiate infection. Proteins of the '''internalin's family''', virulence factors, plays a key role in the infection's survival in a variety of cell types. They play key roles in processes ranging from adhesion to receptor recognition and are thus essential for infection. The internalin family uses a binding partner action. | '''L.monocytogenes''' uses a lot of virulence factors to initiate infection. Proteins of the '''internalin's family''', virulence factors, plays a key role in the infection's survival in a variety of cell types. They play key roles in processes ranging from adhesion to receptor recognition and are thus essential for infection. The internalin family uses a binding partner action. | ||
| Line 26: | Line 33: | ||
== '''Structure of Internalin K''' == | == '''Structure of Internalin K''' == | ||
| + | |||
| + | [[Image:Chain1.png]] | ||
'''Internalin K''' is a multi-domain virulence factor. It harbours four domains formed in the shape of '''"bent arm"'''. | '''Internalin K''' is a multi-domain virulence factor. It harbours four domains formed in the shape of '''"bent arm"'''. | ||
| Line 43: | Line 52: | ||
| + | ===='''Function of internalin K'''==== | ||
| Line 60: | Line 70: | ||
<scene name='56/568014/D2/1'>D2</scene> | <scene name='56/568014/D2/1'>D2</scene> | ||
| - | |||
| - | |||
| - | [[Image:Chain1.png]] | ||
| - | |||
<scene name='56/568014/D3/1'>D3</scene> | <scene name='56/568014/D3/1'>D3</scene> | ||
<scene name='56/568014/D4/1'>D4</scene> | <scene name='56/568014/D4/1'>D4</scene> | ||
Revision as of 19:35, 7 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
Internalin K is a protein from Listeria monocytogene , which is a Gram-positive bacterium human pathogen. It has the ability to survive in the human intestine and to cross a variety of membranes, including mucosal, intestinal, placental, and blood–brain barriers, allows it to generate illnesses ranging from gastroenteritis in healthy individuals to bacteremia and meningitis in immunocompromised patients, as well as mother-to-child infections. Listeria monocytogene can survive in a variety of cell types and proteins of the internalin family have been shown to play a key role in this survival.
Internalin K is involved in Listeria monocytogene ability to escape from autophagy by recruitment of major vault protein to the bacterial surface.
Structure
Internalin family's generalities
Internalin family
L.monocytogenes uses a lot of virulence factors to initiate infection. Proteins of the internalin's family, virulence factors, plays a key role in the infection's survival in a variety of cell types. They play key roles in processes ranging from adhesion to receptor recognition and are thus essential for infection. The internalin family uses a binding partner action.
The three-dimensional structure of the internalin family shows that there are modular proteins in order to improve the binding's partner. A common architecture is pointed, the N-terminal domain, also called N-terminal leucine-rich repeats (LRRs). It is composed of 22-residue regions including a β-strand and an helix. The structure is a curved solenoid. LRR is followed by domains in cell signaling and often in bacterial surface attachment. Whereas the C-terminal regions are not similar that contributes the variety of roles. Each internalin plays a specific role in the infection.
Structure of Internalin K
Internalin K is a multi-domain virulence factor. It harbours four domains formed in the shape of "bent arm".
Domains and are related to domains and by an 90° angle.
The domain composed of a two-helical cap region and also by the leucine-rich repeats (LRRs). It is stably associated to .
has few contacts with which improves the flexibility required by Internalin K to bind to its partner while remaining associated to the surface of the bacterium.
The association of and domains represent the "elbow" region. It means the recognition of major vault protein domain.
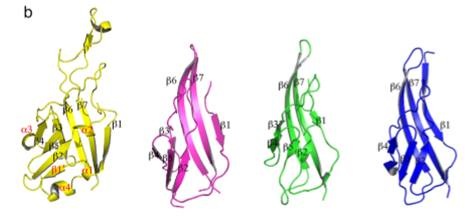
Domains and , located in tandem and related to each other by an approximate 2-fold axis, both fold into compact structures composed of three antiparallel strands packed against two small helices. There are immunoglobulin-like domain.
and are involved in binding to protein partners while and most probably serve as pedestals. The flexibility between domains of its elongated structure may play a key role in this complex function.
Function of internalin K