Introduction
Archaerhodopsin-2 (aR2) is a light-driven proton pump. The resulting proton gradient is subsequently converted into chemical energy.
The structure in 3D represented here is 2Z55, a cristal made of four archaerhodopsin-2.
Archaerhodopsin-2 is composed of 259 amino acids. 88% of this amino acid sequence is identical to the sequence of the archaerhodopsin-1. Moreover, there is 56% identity between this sequence and the sequence of the bacteriorhodopsin. [1]
The trimeric structure of Archaerhodopsin-2
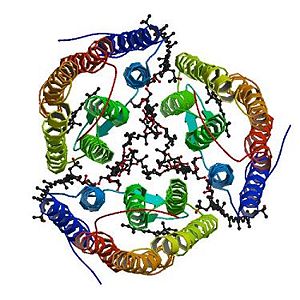
The trimeric structure with its ligands
Archaerhodopsin-2 is a retinal protein–carotenoid complex found in the claret membrane of Halorubrum sp. aus-2 and it represents a real adaptation to life at high salt concentrations. In these membranes, three Archaerhodopsin-2 chains form a trimeric structure [1] (the image on the left side represents the trimeric structure), capturing light energy and using it to move protons across the membrane out of the cell. It exists four different chains with different structures: A,B,D,E (they are not represented here).
The trimerization increases the thermal stability of the protein aR2 in the claret membrane of Halorubrum sp. aus-2 and enlarges the pH range where the protein can keep its neutral conformation. Thus, a larger pH gradient can be generated across the membrane, leading to an increased efficiency of the proton pumping. Therefore the trimeric structure is more efficient than the monomeric structure.
Archaerhodopsin-2 consists of the protein moiety rhodopsin and a reversibly covalently bound cofactor, the retinal.
The trimeric structure functions as a light-driven proton pump thanks to this retinal molecule, called , which changes its conformation when absorbing a photon, resulting in a conformational change of the surrounding protein and the proton pumping action.
Others ligands are linked with each subunit of the trimeric structure like the bacterioruberin (). The bacterioruberin plays a structural role for the trimerization of aR2.
Several saccharides, some lipids and glycolipids also interact with the trimeric structure like the 2,3-di-phytanyl-glycerol (). The lipids and the glycolipids fill the intratrimer hydrophobic space and they are required to the complex activity. Others lipids surround the trimeric structure and are essential to preserve it.[2]
The rhodopsin
The rhodopsin belongs to the CATH Superfamily 1.20.1070.10.[2]The protein has 7 transmembrane alpha helices, embedded in the plasma membrane. These helices are connected to each other by protein loops.
The rhodopsin harvests energy from light to carry out metabolic processes using a non-chlorophyll-based pathway. Thanks to the retinal, the light induces a phototactic response by interacting with transducer membrane-embedded proteins that have no relation to G proteins. There are four different rhodopsins with different structures: A, B, D, E.
Structure and functioning of the Retinal (RET)
The retinal (C20 H28 O) is a photoreactive chromophore.
The rhodopsin binds retinal [3] in a central pocket on the seventh helix by a covalent bond with the . Others bonds exist, like van-der-waals bonds [4].
Retinal is a polyene chromophore and allows to convert light into metabolic energy. It absorbs visible light maximally at 550-570 nm.
It catches a photon, leading to a conformational change of the rhodopsin. This is due to an isomerization of the 11-cis-retinal into a 11-trans-retinal. The retinal binds covalently to the lysine 221 on the transmembrane helix nearest the C-terminus of the protein through a Schiff base linkage. Formation of the Schiff base linkage involves removing the oxygen atom from retinal and two hydrogen atoms from the free amino group of lysine, giving H2O. Retinylidene is the divalent group formed by removing the oxygen atom from retinal, and so opsins is called retinylidene proteins. A Schiff base is a compound with a functional group made up of a carbon-nitrogen double bond with a nitrogen atom connected to an aryl or alkyl group, not hydrogen. Schiff bases in a broad sense have the general formula R1-R2-C=N-R3, where R is an organic side chain. In this definition, Schiff base is synonymous with azomethine. The chain on the nitrogen makes the Schiff base a stable imine. A Schiff base is derived from an aniline, where R3 is a phenyl or a substituted phenyl. Schiff_base in Wikipedia
Ligands
The bacterioruberin (22B)
The bacterioruberin [5] (C50 H76 O4) is a 50 carbon carotenoid pigment which give a red color to the membrane. The primary role of bacterioruberin in the cell is to protect against DNA damage incurred by UV light.[3] This protection is not, however, due to the ability of bacterioruberin to absorb UV light. Bacterioruberin protects the DNA by acting as an antioxidant, rather than directly blocking UV light.[4]It is able to protect the cell from reactive oxygen species produced from exposure to UV by acting as a target.
Furthermore, the bacterioruberin is essential because it plays a structural role for the trimerization of aR2: it mediates interactions between neighbouring monomers.
When the bacterioruberin is bound to the Archaerhodopsin-2, its polyene chain is between the A and B helices of one monomere and the D and E helices of an adjacent one. One end of the bacterioruberin is next to the cytoplasmic membrane surfaces and thus is able to interact with the hydrophilic residus of two monomers. The other end of the bacterioruberin protrudes out of the extracellular membrane. [2]
More precisely, it binds to: the B chain thanks to a hydrogen bond with the , the and thanks to an electrostatic bond with the HOH 304; the D chain thanks to a hydrogen bond with the Tyrosine 156; the E chain thanks to a hydrogen bond with the Tyrosine 156. Others bonds exist like van-der-waals bonds [6].
Glycolipids
Some glycolipids seem to bind to the trimeric structure. They are in the centre of the three monomers and the hydrophilic part seems to be composed of three hexoses connected in tandems.
These glycolipids are supposed to have a stabilizing effect on the trimeric structure because each head group interacts with the two neighbouring monomers. [2]
The 2,3-di-phytanyl-glycerol (L2P)
The 2,3-di-phytanyl-glycerol [7] (C43 H88 O3) is an archaeol (di-O-phytanylglycerol). This is a double ether of sn-1-glycerol where positions 2 and 3 are bound to phytanyl residues. The archaeols are Archaea homologs of diacylglycerols (DAGs).
It interacts with the aR2 surface and the carbohydrate . It binds to: the A chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the ; the B chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the Tyrosine 85; the D chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the Tyrosine 85; the E chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 284 (GLC) and thanks to a hydrogen bond with the Tyrosine 85. Others bonds exist like van-der-waals bonds [8].
Saccharides
Several saccharides can interact with the trimeric structure: β-D-galactose () [9], α-D-glucose () [10] and α-D-mannose () [11].
A molecule of α-D-mannose is covalently bound to one α-D-glucose and one β-D-galactose,thus creating a . There is one ligand by monomer. The glucose can create a covalent bond with the molecule of 2,3-Di-Phytanyl-Glycerol.
Comparison between the Archaerhodopsin-2 and the Bacteriorhodopsin
56% of the Archaeorhodopsin-2 sequence is identical to the Bacteriorhodopsin sequence.[1]
Most amino acids that play a role in the trimerization are not conserved between the two proteins. For instance, the counterparts of some hydrophobic residues of the Archaerhodopsin-2 (the one interacting with the polyene chain of the bacterioruberin) have a different volume. Another difference is the fact that the hydrophobic residues of the Archaerhodopsin-2 (responsible for the hydrogen bonds with the bacterioruberin) are replaced in the Bacteriorhodopsin by polar amino acids.
However the global structures of Archaeorhodopsin-2 and Bacteriorhodopsin are really similar, especially at the level of the open space between the monomers. The interaction between the monomers of the Bacteriorhodopsin is also mediated by lipids: diphytanyl diether phospholipids instead of Bacterioruberin.
This similarity of structure forms the basis of several hypothesis concerning the mechanisms of the Archaeorhodopsin-2 [2]

