Sandbox Reserved 817
From Proteopedia
| Line 61: | Line 61: | ||
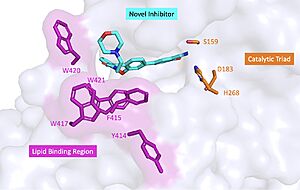
| - | [[Image: | + | [[Image:Inhibitor.jpg|right|300px|thumb|'''Figure 2 :''' Docking study on compound 4 bound to BACE1. The coordinates of BACE1 was taken from the crystal structure of 1FKN. The protein is shown in cartoon, while the important residues and ligand 4 are shown in stick model.'']] |
In the past, major efforts in designing BACE1 inhibitors were focused on the transition state analogs such as hydroxyethylamines, hydroxyethylene, and tatine-based peptidomimetic inhibitors but their relatively large sizes and excessive number of hydrogen-bond donors and acceptors made it difficult for them to penetrate the blood brain barrier. Therefore the scientific community focused on other coompounds. | In the past, major efforts in designing BACE1 inhibitors were focused on the transition state analogs such as hydroxyethylamines, hydroxyethylene, and tatine-based peptidomimetic inhibitors but their relatively large sizes and excessive number of hydrogen-bond donors and acceptors made it difficult for them to penetrate the blood brain barrier. Therefore the scientific community focused on other coompounds. | ||
Revision as of 19:07, 9 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
BACE1
| |||||||
| Crystal structure of BACE1 with its inhibitor, 4ivs | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Activity: | Memapsin 2, with EC number 3.4.23.46 | ||||||
| Related: | 4ivt
| ||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Contents |
Introduction
BACE1, which stands for β-site of Amyloid precursor protein Cleaving Enzyme 1, is a β secretase also known as memapsin 2. It is an aspartyl protease that initiates the formation of β-amyloid which accumulation has a huge role in the Alzeimer disease. It had been brought to light that not only BACE1 levels rise in the brain of the patients suffering from this disease but that BACE1 knock out mices can’t produce β-amyloid, effectively preventing the formation of amyloid plaques and the development of the Alzheimer disease. This discovery makes the BACE1 a very interesting drug target for Alzheimer disease.The inhibition of this enzyme is a plausible course of action and several inhibitors like the ones that will be presented are considered. However, the total inhibition of BACE1 seems to leads to physiological and behavioural troubles. That would mean that BACE1 could also be involved in several other brain functions. So the regulation of BACE1 activity by partial inhibition seems to be a prefencial method. Anyway, a good understanding of the biological function, the structure and the mechanisms of inhibition of BACE1 is highly required for drug design.
Localisation and Biological function
Localisation
BACE1 is the result of the expression of the BACE1 gene localised on chromosome 11 (30kb). This gene is composed of 9 exons that are linked together during the mRNA maturation. BACE1 is expressed in the majority of human tissues however, the highest levels of activity are found in the neuronal cell. This β secretase is a transmembrane protein mainly localised within cholesterol-rich lipid raft. It is found in the acidic compartments of the secretory pathway (mostly endosomes and the Trans Golgi Network) with its catalytic site facing the lumen side. This enzyme is regulated both at the translation and transcription steps and its expression is correlated with cell stress signals. [1]
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Cole SL, Vassar R. The Alzheimer's disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007 Nov 15;2:22. PMID:18005427 doi:http://dx.doi.org/10.1186/1750-1326-2-22
- ↑ Haniu M, Denis P, Young Y, Mendiaz EA, Fuller J, Hui JO, Bennett BD, Kahn S, Ross S, Burgess T, Katta V, Rogers G, Vassar R, Citron M. Characterization of Alzheimer's beta -secretase protein BACE. A pepsin family member with unusual properties. J Biol Chem. 2000 Jul 14;275(28):21099-106. PMID:10887202 doi:10.1074/jbc.M002095200
- ↑ 3.0 3.1 Zou Y, Li L, Chen W, Chen T, Ma L, Wang X, Xiong B, Xu Y, Shen J. Virtual screening and structure-based discovery of indole acylguanidines as potent beta-secretase (BACE1) inhibitors. Molecules. 2013 May 16;18(5):5706-22. doi: 10.3390/molecules18055706. PMID:23681056 doi:http://dx.doi.org/10.3390/molecules18055706
- ↑ Dislich B, Lichtenthaler SF. The Membrane-Bound Aspartyl Protease BACE1: Molecular and Functional Properties in Alzheimer's Disease and Beyond. Front Physiol. 2012;3:8. doi: 10.3389/fphys.2012.00008. eCollection 2012. PMID:22363289 doi:http://dx.doi.org/10.3389/fphys.2012.00008
Proteopedia page contributors and editors
DANTAN Gaëlle and MAHLER-WOHLGEMUTH Adrien
Student 1A ESBS (Promo 2016)