Sandbox Reserved 814
From Proteopedia
(Difference between revisions)
(→RIBOSOMAL PROTEIN L14) |
|||
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
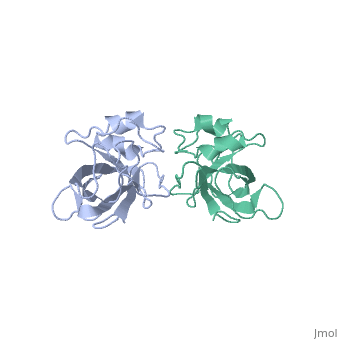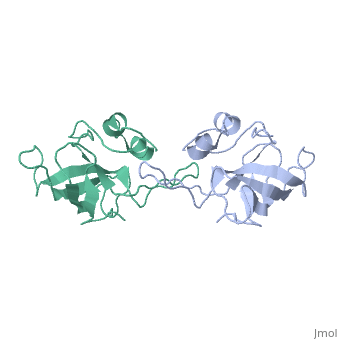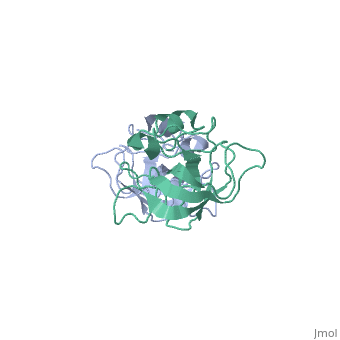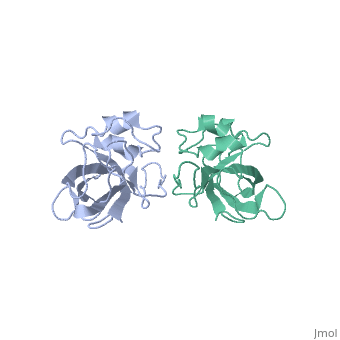
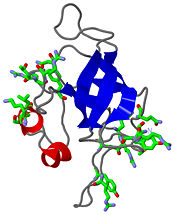
[[Image:L14structure.jpg|175px|left|thumb| Geobacillus stearothermophilus's L14 ribosomal protein structure.]] | [[Image:L14structure.jpg|175px|left|thumb| Geobacillus stearothermophilus's L14 ribosomal protein structure.]] | ||
| - | The L14 ribosomal protein is a protein which is included in the 50S subunit of the procaryote ribosome and in the 60s subunit of the eucaryote ribosome.The L14 ribosomal protein take part in | + | The L14 ribosomal protein is a protein which is included in the 50S subunit of the procaryote ribosome and in the 60s subunit of the eucaryote ribosome.The L14 ribosomal protein take part in an operon in Procaryotic and on the third human's chromosome. The 50S and 60S subunits are the twos biggest subunits of the prokaryotic and eukaryotic ribosomes. It is a cytoplasmic protein which contains a basic region leucine zipper which participates to the mechanism of translation by allowing the folding and stabilization of rRNA. |
| - | The L14 subunit is one of the most conserved protein in the ribosome in a lot species. For example, 66% sequence identity exists between the Escherichia coli and Bacillus stearothermophilus molecules. L14 has been located | + | The L14 subunit is one of the most conserved protein in the ribosome in a lot of species. For example, 66% sequence identity exists between the Escherichia coli and Bacillus stearothermophilus molecules. L14 has been located on the 50S and 30S subunit interface between peptidyl transferase and GTPase regions. It allows contacts with the 16S rRNA of the 30S subunit (bridges B5 and B8) connecting the 2 subunits. |
== Structure == | == Structure == | ||
| Line 21: | Line 21: | ||
<Structure load='3cc2' size='300' frame='true' align='center' caption='All ribosomal proteins allow the stabilization of the rRNA.' scene='Insert optional scene name here' /> | <Structure load='3cc2' size='300' frame='true' align='center' caption='All ribosomal proteins allow the stabilization of the rRNA.' scene='Insert optional scene name here' /> | ||
| + | |||
| + | == References == | ||
| + | |||
| + | Christopher Davies, Stephen W White, V Ramakrishnan. The crystal structure of ribosomal protein L14 reveals an important organizational component of the translational apparatus. | ||
By Bonhomme Clémence and Dilda Nathan</StructureSection><!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | By Bonhomme Clémence and Dilda Nathan</StructureSection><!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | ||
{{Sandbox_Reserved_ESBS}} | {{Sandbox_Reserved_ESBS}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
Revision as of 20:48, 9 January 2014
RIBOSOMAL PROTEIN L14
| |||||||||||
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |