Hexokinase
From Proteopedia
| Line 1: | Line 1: | ||
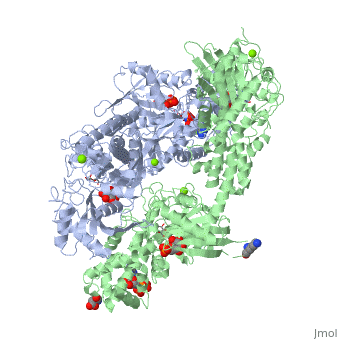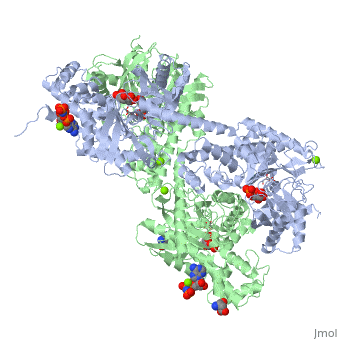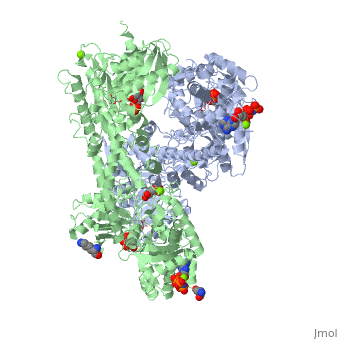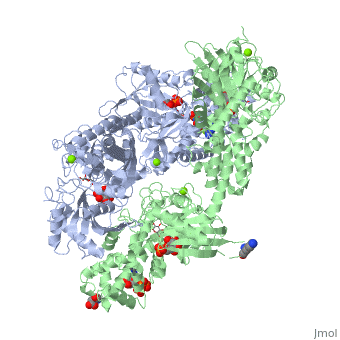
| - | <StructureSection load='1qha' size='450' side='right' scene='' caption=''> | + | <StructureSection load='1qha' size='450' side='right' scene='' caption='Hexokinase I complex with ATP analog, glucose, glucose-phosphate and Mg+2 ion (PDB code [[1qha]])'> |
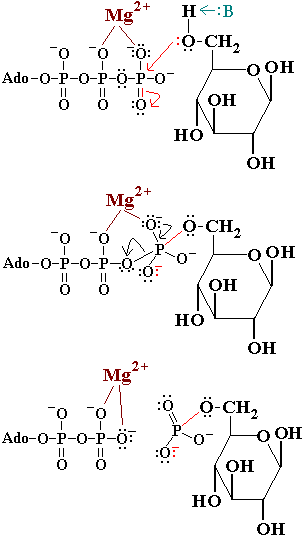
'''Hexokinase''' is an enzyme that phosphorylates a six-carbon sugar, a hexose, to a hexose phosphate. In most tissues and organisms, glucose is the most important substrate of hexokinases, and glucose 6-phosphate the most important product. Hexokinases have been found in every organism checked, ranging from bacteria, yeast, and plants, to humans and other vertebrates. They are categorized as actin fold proteins, sharing a common ATP binding site core surrounded by more variable sequences that determine substrate affinities and other properties. Several hexokinase isoforms or isozymes providing different functions can occur in a single species. | '''Hexokinase''' is an enzyme that phosphorylates a six-carbon sugar, a hexose, to a hexose phosphate. In most tissues and organisms, glucose is the most important substrate of hexokinases, and glucose 6-phosphate the most important product. Hexokinases have been found in every organism checked, ranging from bacteria, yeast, and plants, to humans and other vertebrates. They are categorized as actin fold proteins, sharing a common ATP binding site core surrounded by more variable sequences that determine substrate affinities and other properties. Several hexokinase isoforms or isozymes providing different functions can occur in a single species. | ||
Revision as of 10:54, 13 February 2014
| |||||||||||
3D structures of hexokinase
Updated on 13-February-2014
3o08, 3o1b, 3o1w, 3o4w, 3o6w, 3o80, 4jax – KlHK – Kluyveromyces lactis
2e2n – StHK – Sulfolobus tokodaii
2e2o - StHK + glucose
2e2p – StHK + ADP
2e2q - StHK + ADP + xylose + Mg
3o5b, 3o8m – KlHK + glucose
1bdg – HK + glucose – Schistosoma mansoni
Hexokinase I
3b8a – yHK I + glucose – yeast
1hkg – yHK I
1dgk – hHK I (mutant) + ADP + glucose – human
1cza - hHK I (mutant) + ADP + glucose-6-phosphate + glucose
1bg3 - HK I + glucose-6-phosphate + glucose - rat
1qha – hHK I + AMP-PNP
1hkc - hHK I + phosphate + glucose
1hkb, 4f9o - hHK I + glucose-6-phosphate + glucose
4fpa - hHK I (mutant) + glucose-6-phosphate + glucose
4foe - hHK I + mannose-6-phosphate + glucose
4foi - hHK I (mutant) + glucose 1,6-bisphosphate + glucose
4fpb - hHK I + 1,5-anhydroglucitol-6-phosphate + glucose
Hexokinase II
1ig8, 2yhx – yHK II
2nzt – hHK II
Hexokinase III
3hm8 – hHK III C terminal
Hexokinase IV (Glucokinase GK)
3qic – hHK IV residues 12-465 (mutant)
1v4t – hGK
3mcp – GK – Parabacterioides distasonis
2qm1 – GK – Enterococcus faecalis
3vgk – SgGK – Streptomyces griseus
1q18 – EcGK – Escherichia coli
4eun – GK – Janibacter
3vov – GK – Thermus thermophilus
Hexokinase IV binary complex
1sz2 - EcGK + glucose
3vgm - SgGK + glucose
3idh - hHK IV residues 12-465 + glucose
3h1v, 3imx, 3a0i, 3goi,1v4s, 3s41, 3vev, 3vf6, 4dch, 4dhy - hHK IV residues 12-465 + synthetic activator
3fr0, 4l3q, 4ise, 4isf, 4isg, 4iwv, 4ixc - hHK IV residues 12-465 + activator
Hexokinase IV ternary complex
3id8, 3fgu - hHK IV residues 12-465 + AMP-PNP + glucose
3f9m - hHK IV residues 12-465 + activator + glucose
2q2r - GK + glucose + ADP – Trypanosoma cruzi
3vgl - SgGK + glucose + AMP-PNP
3vey - hGK + glucose + ATPgS
ADP-dependent GK
1gc5 – AGK + ADP – Thermococcus litoralis
1l2l – AGK – Pyrococcus horikoshii
1ua4 - AGK – Pyrococcus furiosus
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
1.↑ Pollard-Knight D, Cornish-Bowden A. Mechanism of liver glucokinase. Mol Cell Biochem. 1982 Apr 30;44(2):71-80. PMID:7048063
2.↑ 2.0 2.1 Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004 Mar;12(3):429-38. PMID:15016359 doi:10.1016/j.str.2004.02.005
3.↑ Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog Horm Res. 2001;56:195-217. PMID:11237213
4.↑ Zeng C, Aleshin A, Hardie J, Harrison R, Fromm H. ATP-Binding site of Human Brain Hexokinase as Studied by Molecular Modeling and Site-Directed Mutagenesis. Biochem. 1996 Aug 6;35:13157-13164.
5.↑ hammes G, and Kochavi D. Studies of the Enzyme Hexokinase: Kinetic Inhibition by Products. Massachusetts Institute of Technology. 1961 Oct 5.
6.↑ Ralph E, Thomson J, Almaden J, Sun S. Glucose Modulation fo Glucokinase Activation by Small Molecules. Biochem. 2008 Feb 15;47:5028-5036.
7.↑ Pal P, and Miller B. Activating Mutations in the Human Glucokinase Gene Revealed by Genetic Selection. Biochem. 2008 Dec 3;48:814-816.
8.↑ Aleshin A, Malfois M, Liu X, Kim C, Fromm H, Honzatko R, Koch M, Svergun D. Nonaggregating Mutant of Recombinant Human Hexokinase I Exhibits Wild-Type Kinetics and Rod-like Conformations in Solution. Biochem. 1999 Apr 29;38:8359-8366.
Seth Bawel and Kyle_Schroering created this page in Che 361 at Wabash College.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Ann Taylor, Alexander Berchansky