This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
3hts
From Proteopedia
(New page: 200px<br /><applet load="3hts" size="450" color="white" frame="true" align="right" spinBox="true" caption="3hts, resolution 1.75Å" /> '''HEAT SHOCK TRANSCRIP...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:3hts.jpg|left|200px]]<br /><applet load="3hts" size=" | + | [[Image:3hts.jpg|left|200px]]<br /><applet load="3hts" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="3hts, resolution 1.75Å" /> | caption="3hts, resolution 1.75Å" /> | ||
'''HEAT SHOCK TRANSCRIPTION FACTOR/DNA COMPLEX'''<br /> | '''HEAT SHOCK TRANSCRIPTION FACTOR/DNA COMPLEX'''<br /> | ||
==Overview== | ==Overview== | ||
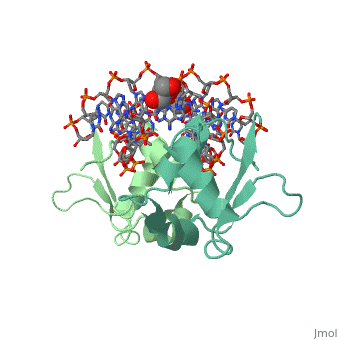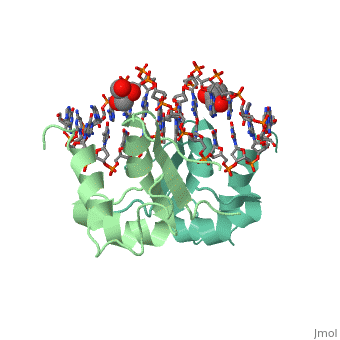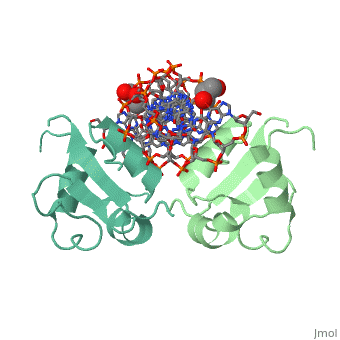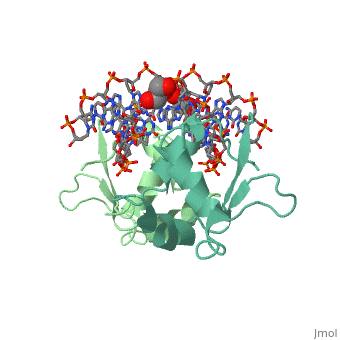
| - | The 1.75 A crystal structure of the Kluyveromyces lactis heat shock | + | The 1.75 A crystal structure of the Kluyveromyces lactis heat shock transcription factor (HSF) DNA-binding domain (DBD) complexed with DNA reveals a protein-DNA interface with few direct major groove contacts and a number of phosphate backbone contacts that are primarily water-mediated interactions. The DBD, a 'winged' helix-turn-helix protein, displays a novel mode of binding in that the 'wing' does not contact DNA like all others of that class. Instead, the monomeric DBD, which crystallized as a symmetric dimer to a pair of nGAAn inverted repeats, uses the 'wing' to form part of the protein-protein contacts. This dimer interface is likely important for increasing the DNA-binding specificity and affinity of the trimeric form of HSF, as well as for increasing cooperativity between adjacent trimers. |
==About this Structure== | ==About this Structure== | ||
| - | 3HTS is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Kluyveromyces_lactis Kluyveromyces lactis] with GOL as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http:// | + | 3HTS is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Kluyveromyces_lactis Kluyveromyces lactis] with <scene name='pdbligand=GOL:'>GOL</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3HTS OCA]. |
==Reference== | ==Reference== | ||
| Line 14: | Line 14: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Littlefield, O.]] | [[Category: Littlefield, O.]] | ||
| - | [[Category: Nelson, H | + | [[Category: Nelson, H C.M.]] |
[[Category: GOL]] | [[Category: GOL]] | ||
[[Category: complex (winged helix_turn_ helix/dna)]] | [[Category: complex (winged helix_turn_ helix/dna)]] | ||
| Line 20: | Line 20: | ||
[[Category: transcription regulation]] | [[Category: transcription regulation]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 19:09:50 2008'' |
Revision as of 17:09, 21 February 2008
|
HEAT SHOCK TRANSCRIPTION FACTOR/DNA COMPLEX
Overview
The 1.75 A crystal structure of the Kluyveromyces lactis heat shock transcription factor (HSF) DNA-binding domain (DBD) complexed with DNA reveals a protein-DNA interface with few direct major groove contacts and a number of phosphate backbone contacts that are primarily water-mediated interactions. The DBD, a 'winged' helix-turn-helix protein, displays a novel mode of binding in that the 'wing' does not contact DNA like all others of that class. Instead, the monomeric DBD, which crystallized as a symmetric dimer to a pair of nGAAn inverted repeats, uses the 'wing' to form part of the protein-protein contacts. This dimer interface is likely important for increasing the DNA-binding specificity and affinity of the trimeric form of HSF, as well as for increasing cooperativity between adjacent trimers.
About this Structure
3HTS is a Single protein structure of sequence from Kluyveromyces lactis with as ligand. Full crystallographic information is available from OCA.
Reference
A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal., Littlefield O, Nelson HC, Nat Struct Biol. 1999 May;6(5):464-70. PMID:10331875
Page seeded by OCA on Thu Feb 21 19:09:50 2008