We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 916
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
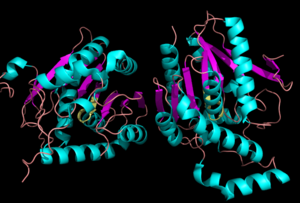
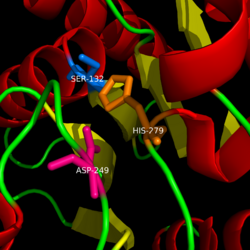
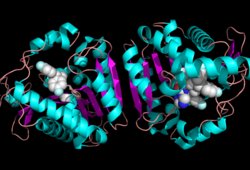
Monoglyceride lipase is part of the α/β hydrolase family, having a Ser-His-Asp catalytic triad (Celemnte et al. 2012). MGL terminates the signaling of a primary endocannabinoid, 2-AG (Savinainen et al 2010). MGL is able to hydrolyze 2-arachidonoylglycerol into arachidonic acid and glycerol (Bertrand et al. 2010). One of the key features of MGL is the hydrophobic tunnel, which has been suggested to provide a model for drug research. | Monoglyceride lipase is part of the α/β hydrolase family, having a Ser-His-Asp catalytic triad (Celemnte et al. 2012). MGL terminates the signaling of a primary endocannabinoid, 2-AG (Savinainen et al 2010). MGL is able to hydrolyze 2-arachidonoylglycerol into arachidonic acid and glycerol (Bertrand et al. 2010). One of the key features of MGL is the hydrophobic tunnel, which has been suggested to provide a model for drug research. | ||
| - | ===Metabolic Role=== | + | ===Metabolic Role=== |
| + | Monoglyceride lipase is able to hydrolyze monoacylglycerols into fatty acids and glycerol (Taschler et al. 2011). MGL degrades sn-1 and 2-MG at identical specific rates as a part of its metabolic role (Taschler et al. 2011). | ||
| + | |||
| + | ===Component of Endocannabinoid System=== | ||
| + | MGL degrades 2-arachidonoyl glycerol (2-AG). 2-AG is commonly classified as an endocannabinoid. In the brain endocannabinoids are released from postsynaptic neurons, causing the retrograde suppression of synaptic transmission (Taschler et al. 2011). | ||
| + | In Peripheral tissues, EC is active in autonomic nervous system. EC affects processes such as learning, motor control, cognition, and pain (Taschler et al. 2011). EC is also able to regulate lipid metabolism and food intake (Taschler et al. 2011). | ||
| + | Clinical Relevance: Taschler et al. looked at the role of MGL in energy metabolism, finding that MGL deficiency in animals led to the buildup of 2-AG (Taschler et al. 2011). | ||
| - | ===Component of Endocannabinoid System=== | ||
==Structure== | ==Structure== | ||
| Line 28: | Line 33: | ||
== References == | == References == | ||
| + | Savinainen, Juha R., Megumi Yoshino, Anna Minkkilä, Tapio Nevalainen, and Jarmo T. Laitinen. "Characterization of Binding Properties of Monoglyceride Lipase Inhibitors by a Versatile Fluorescence-based Technique." Analytical Biochemistry 399.1 (2010): 132-34 | ||
<references/> | <references/> | ||
Revision as of 12:11, 25 March 2014
Monoglyceride Lipase (MGL)
| |||||||||||