We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daud Akhtar/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
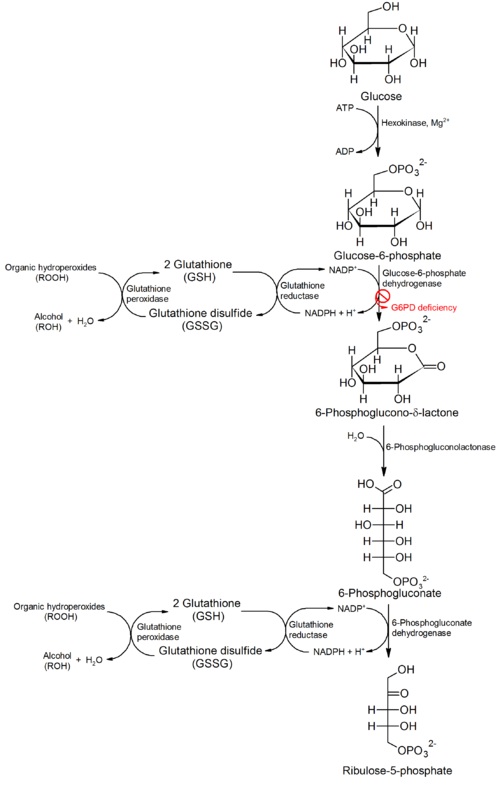
The major catabolic fate of G6P is glycolytic breakdown through glycolysis. Another fate however is the oxidation of G6P into pentose phosphates through the pentose phosphate pathway. The first reaction of the pentose phosphate pathway is the oxidation of G6P by G6PD to form 6-phosphoglucono-δ-lactone. NADP+ is the electron acceptor, and the overall equilibrium lies far in the direction of NADPH formation. In this pathway, G6PD is the rate-limiting enzyme. Biologically, the pentose phosphate pathway plays an important role in generating NADPH. High levels of NADP+ in the cytosol act as an allosteric stimulator of G6PD by promoting G6P usage as a substrate in the pentose phosphate pathway. The ultimate product of the pentose phosphate pathway is ribose-5- phosphate. Rapidly dividing cells, such as those of bone marrow, skin, and tumors use ribose-5-phosphate to make RNA, DNA, and coenzymes such as ATP, NADH, and FADH2. In other tissues, the essential product of the pentose phosphate pathway is not the pentose products but the electron donors NADPH. NADPH is needed for reductive biosynthesis or to counter the damaging effects of oxygen radicals. Additionally tissues that are heavily involved in fatty acid synthesis such as liver and adipose tissue, require NADPH to fuel the synthesis of cholesterols and steroid hormones. In the case of erythrocytes and cells, which are directly exposed to oxygen such as the cornea, maintain a high ratio of NADPH/NADP+ and glutathione to prevent or undo oxidative damage caused by the generation of free radicals. A genetic defect in G6PD is known as G6PD Deficiency and can have serious medical consequences. | The major catabolic fate of G6P is glycolytic breakdown through glycolysis. Another fate however is the oxidation of G6P into pentose phosphates through the pentose phosphate pathway. The first reaction of the pentose phosphate pathway is the oxidation of G6P by G6PD to form 6-phosphoglucono-δ-lactone. NADP+ is the electron acceptor, and the overall equilibrium lies far in the direction of NADPH formation. In this pathway, G6PD is the rate-limiting enzyme. Biologically, the pentose phosphate pathway plays an important role in generating NADPH. High levels of NADP+ in the cytosol act as an allosteric stimulator of G6PD by promoting G6P usage as a substrate in the pentose phosphate pathway. The ultimate product of the pentose phosphate pathway is ribose-5- phosphate. Rapidly dividing cells, such as those of bone marrow, skin, and tumors use ribose-5-phosphate to make RNA, DNA, and coenzymes such as ATP, NADH, and FADH2. In other tissues, the essential product of the pentose phosphate pathway is not the pentose products but the electron donors NADPH. NADPH is needed for reductive biosynthesis or to counter the damaging effects of oxygen radicals. Additionally tissues that are heavily involved in fatty acid synthesis such as liver and adipose tissue, require NADPH to fuel the synthesis of cholesterols and steroid hormones. In the case of erythrocytes and cells, which are directly exposed to oxygen such as the cornea, maintain a high ratio of NADPH/NADP+ and glutathione to prevent or undo oxidative damage caused by the generation of free radicals. A genetic defect in G6PD is known as G6PD Deficiency and can have serious medical consequences. | ||
| - | [[Image: | + | [[Image:Pathology of G6PD deficiency.jpg]] |
| + | |||
Revision as of 02:26, 30 March 2014
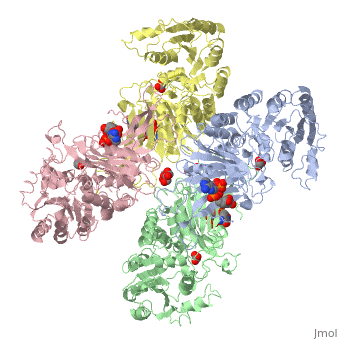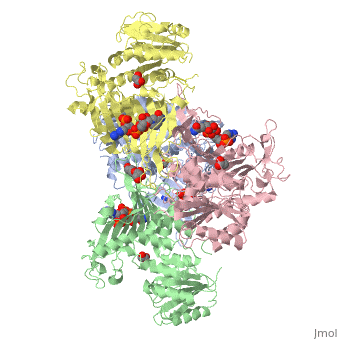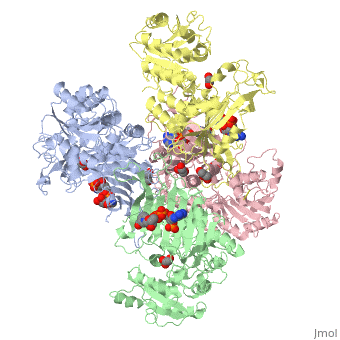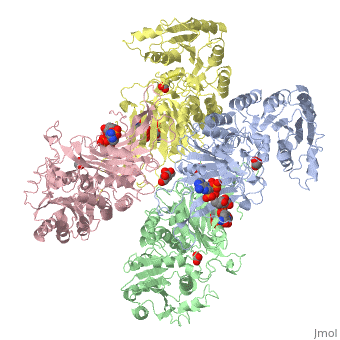
Glucose-6-Phosphate Dehydrogenase(G6PD)
| |||||||||||
Glucose 6 Phosphate Dehydrognease
jghgjgjhgjhg
References
- ↑ Salati LM, Amir-Ahmady B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr. 2001;21:121-40. PMID:11375432 doi:http://dx.doi.org/10.1146/annurev.nutr.21.1.121
- ↑ Salati LM, Amir-Ahmady B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr. 2001;21:121-40. PMID:11375432 doi:http://dx.doi.org/10.1146/annurev.nutr.21.1.121
- ↑ . Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67(6):601-11. PMID:2633878
- ↑ Au SW, Gover S, Lam VM, Adams MJ. Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure. 2000 Mar 15;8(3):293-303. PMID:10745013
- ↑ Manganelli G, Masullo U, Passarelli S, Filosa S. Glucose-6-phosphate dehydrogenase deficiency: disadvantages and possible benefits. Cardiovasc Hematol Disord Drug Targets. 2013 Mar 1;13(1):73-82. PMID:23534950
- ↑ Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 1991 Jan 17;324(3):169-74. PMID:1984194 doi:http://dx.doi.org/10.1056/NEJM199101173240306