We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 920
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
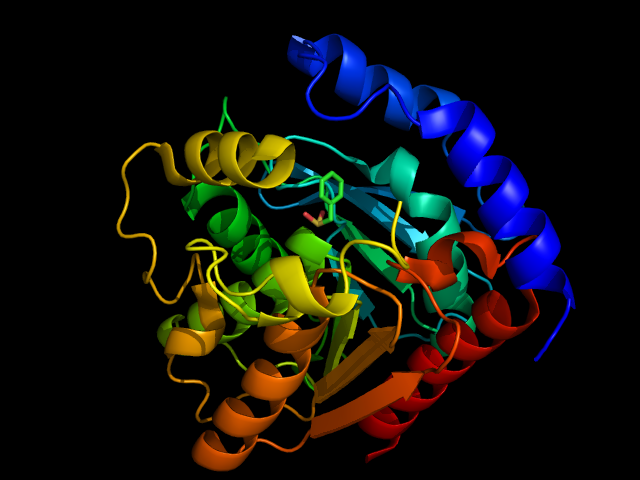
The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads | The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads | ||
http://www.piercenet.com/product/pmsf-phenylmethylsulfonyl-fluoride | http://www.piercenet.com/product/pmsf-phenylmethylsulfonyl-fluoride | ||
| + | |||
| + | |||
| + | Hormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride (PMSF) entering into the active site. PMSF inhibits enzymes by binding to the serine residue of the serine protease active sites so that the normal catalytic activity cannot be carried out. This inhibitor will only bind to the active site serine because of its increased activity from being in the catalytic triad. | ||
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
<references /> | <references /> | ||
Revision as of 23:34, 30 March 2014
Your Heading Here (maybe something like 'Structure')
Anything in this section will appear adjacent to the 3D structure and will be scrollable. This is a default text for your page Sandbox reserved 920
Structure
| |||||||||||