We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daud Akhtar/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1qki' size='340' side='right' caption='Glucose 6 Phosphate Dehydrogenase(1QKI) Structure' scene=''> | <StructureSection load='1qki' size='340' side='right' caption='Glucose 6 Phosphate Dehydrogenase(1QKI) Structure' scene=''> | ||
==Introduction== | ==Introduction== | ||
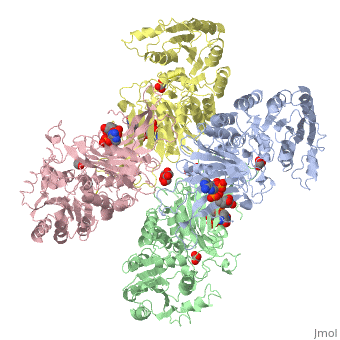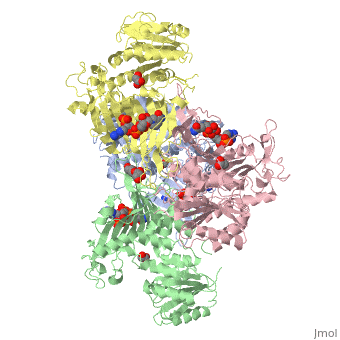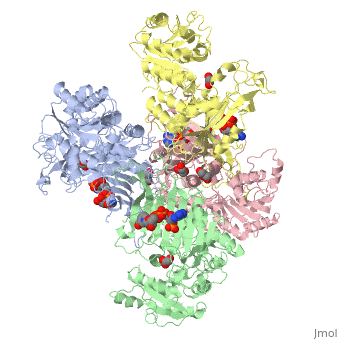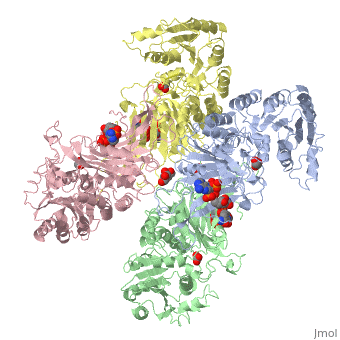
| - | Glucose 6-Phosphate Dehydrogenase (G6PD) is a metabolic X-linked enzyme, which catalyzes the conversion of glucose-6-phosphate (G6P) to 6-phosphoglucono-δ-lactone<ref>PMID: 11375432 </ref>. This redox reaction is the first and rate-determining step in the pentose phosphate pathway in which coenzyme NADP+ is reduced through the transfer of a hydride from G6P | + | Glucose 6-Phosphate Dehydrogenase (G6PD) is a metabolic X-linked enzyme, which catalyzes the conversion of glucose-6-phosphate (G6P) to 6-phosphoglucono-δ-lactone<ref>PMID: 11375432 </ref>. This redox reaction is the first and rate-determining step in the pentose phosphate pathway in which coenzyme NADP+ is reduced through the transfer of a hydride from G6P <ref name="1qki">PMID: 10745013 </ref> Consequently, NADPH is generated and helps restore reduced glutathione (GSH), which is an important anti-oxidant. NADPH and GSH thereby help protect cells from oxidative stress by converting peroxides into water<ref>PMID: 15858258 </ref>. G6PD is most abundant in intracellular fluid and is conserved over a large array of different organisms. Specifically, higher plants exhibit several isoforms of G6PD in different cellular locations such as the cytosol, the plastidic stroma, and peroxisomes <ref>PMID: 9480890 </ref>. In humans, active G6PD generally exists as a dimer/tetramer equilibrium. More than 400 different variants of G6PD have been identified where these variants differ in the location of point mutations in the G6PD gene. The G6PD gene is located on the chromosome Xq28 region<ref name="1qki" />. These mutations result in deficiencies which range from mild to severe phenotypic abnormalities such as hemolytic anemia. The exposure of erythrocytes to oxidative stress due to a lack of NADPH in cells results in their destruction which causes hemolytic anemia.<ref name="1qki" />. G6PD is involved in the binding of <scene name='58/580852/Ligand/4'>ligands(both G6P and coenzyme NADP+) on each respective subunit of a G6PD tetramer(ABCD) where ligand color corresponds to the respective subunit(A=blue,B=green,C=pink,D=yellow) </scene> indicating a structure that implies a function involving metabolic pathways. |
==Species Distribution== | ==Species Distribution== | ||
Revision as of 00:45, 31 March 2014
Glucose-6-Phosphate Dehydrogenase(G6PD)
| |||||||||||
References
- ↑ Salati LM, Amir-Ahmady B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr. 2001;21:121-40. PMID:11375432 doi:http://dx.doi.org/10.1146/annurev.nutr.21.1.121
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Au SW, Gover S, Lam VM, Adams MJ. Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure. 2000 Mar 15;8(3):293-303. PMID:10745013
- ↑ Kotaka M, Gover S, Vandeputte-Rutten L, Au SW, Lam VM, Adams MJ. Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr. 2005 May;61(Pt 5):495-504. Epub 2005, Apr 20. PMID:15858258 doi:http://dx.doi.org/10.1107/S0907444905002350
- ↑ Corpas FJ, Barroso JB, Sandalio LM, Distefano S, Palma JM, Lupianez JA, Del Rio LA. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem J. 1998 Mar 1;330 ( Pt 2):777-84. PMID:9480890
- ↑ Au SW, Naylor CE, Gover S, Vandeputte-Rutten L, Scopes DA, Mason PJ, Luzzatto L, Lam VM, Adams MJ. Solution of the structure of tetrameric human glucose 6-phosphate dehydrogenase by molecular replacement. Acta Crystallogr D Biol Crystallogr. 1999 Apr;55(Pt 4):826-34. PMID:10089300
- ↑ Bhadbhade MM, Adams MJ, Flynn TG, Levy HR. Sequence identity between a lysine-containing peptide from Leuconostoc mesenteroides glucose-6-phosphate dehydrogenase and an active site peptide from human erythrocyte glucose-6-phosphate dehydrogenase. FEBS Lett. 1987 Jan 26;211(2):243-6. PMID:3100332
- ↑ Cosgrove MS, Naylor C, Paludan S, Adams MJ, Levy HR. On the mechanism of the reaction catalyzed by glucose 6-phosphate dehydrogenase. Biochemistry. 1998 Mar 3;37(9):2759-67. PMID:9485426 doi:10.1021/bi972069y
- ↑ Ramos KL, Colquhoun A. Protective role of glucose-6-phosphate dehydrogenase activity in the metabolic response of C6 rat glioma cells to polyunsaturated fatty acid exposure. Glia. 2003 Aug;43(2):149-66. PMID:12838507 doi:http://dx.doi.org/10.1002/glia.10246
- ↑ Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem. 1998 Apr 24;273(17):10609-17. PMID:9553122
- ↑ Scott MD, Zuo L, Lubin BH, Chiu DT. NADPH, not glutathione, status modulates oxidant sensitivity in normal and glucose-6-phosphate dehydrogenase-deficient erythrocytes. Blood. 1991 May 1;77(9):2059-64. PMID:2018843
- ↑ Scott MD, Zuo L, Lubin BH, Chiu DT. NADPH, not glutathione, status modulates oxidant sensitivity in normal and glucose-6-phosphate dehydrogenase-deficient erythrocytes. Blood. 1991 May 1;77(9):2059-64. PMID:2018843
- ↑ . Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67(6):601-11. PMID:2633878
- ↑ Manganelli G, Masullo U, Passarelli S, Filosa S. Glucose-6-phosphate dehydrogenase deficiency: disadvantages and possible benefits. Cardiovasc Hematol Disord Drug Targets. 2013 Mar 1;13(1):73-82. PMID:23534950
- ↑ Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 1991 Jan 17;324(3):169-74. PMID:1984194 doi:http://dx.doi.org/10.1056/NEJM199101173240306