Sandbox Reserved 918
From Proteopedia
| Line 9: | Line 9: | ||
===Introduction=== | ===Introduction=== | ||
| - | '''Dipeptidyl Peptidase IV''' (commonly abbreviated as '''DPP IV''') is a regulatory [http://en.wikipedia.org/wiki/Protease protease] and binding [http://en.wikipedia.org/wiki/Glycoprotein glycoprotein] that carries out numerous functions in humans making it a prime candidate for medicinal and pharmaceutical research. DPP IV, discovered by V.K. Hopsu-Havu and G.G. Glenner in homogenized rat liver tissue, was originally believed to serve a specific role in breaking [http://en.wikipedia.org/wiki/2-Naphthylamine 2-Naphthylamine] off of [http://brenda-enzymes.org/php/ligand_flatfile.php4?brenda_ligand_id=228740 Gly-Pro-2-napthylamide], hence its original name glycylproline napthylamidase. However, further research into the specificity of DPP IV eventually showed that it serves a more generic function as a [http://en.wikipedia.org/wiki/Hydrolase hydrolase] (a serine [http://en.wikipedia.org/wiki/Exopeptidase exopeptidase]), breaking N-terminal Xaa-Pro bonds (though it can alΌso catalyze alanine bonds). DPP IV is the founding member of the DPP-IV and/or structure homologue (DASH) family, who all share this serine protease catalysis of post-proline peptide bonds. <ref> PMID: 16186403</ref> These penultimate [http://en.wikipedia.org/wiki/Proline prolines] of the [http://en.wikipedia.org/wiki/N-terminus N-terminus] are known for their ability to resist attacks from most proteases and also induce a [http://en.wikipedia.org/wiki/Conformational_change conformational change] of their respective proteins. Also, DPP IV serves as a binding glycoprotein on the membrane of cells, binding ligands such as [http://en.wikipedia.org/wiki/Adenosine_deaminase adenosine deaminase] with high affinity.<ref name="Gorrell"/> Though this interaction has no known significance as of yet, DPP IV and its ability to catalyze N-terminal prolines gives it a unique specificity and target for pharmaceutical companies to take advantage of. <ref name="regpeps">PMID: 10588446</ref> | + | '''Dipeptidyl Peptidase IV''' (commonly abbreviated as '''DPP IV''') is a regulatory [http://en.wikipedia.org/wiki/Protease protease] and binding [http://en.wikipedia.org/wiki/Glycoprotein glycoprotein] that carries out numerous functions in humans making it a prime candidate for medicinal and pharmaceutical research. DPP IV, discovered by V.K. Hopsu-Havu and G.G. Glenner in homogenized rat liver tissue<ref name="regpeps">PMID: 10588446</ref>, was originally believed to serve a specific role in breaking [http://en.wikipedia.org/wiki/2-Naphthylamine 2-Naphthylamine] off of [http://brenda-enzymes.org/php/ligand_flatfile.php4?brenda_ligand_id=228740 Gly-Pro-2-napthylamide], hence its original name glycylproline napthylamidase. However, further research into the specificity of DPP IV eventually showed that it serves a more generic function as a [http://en.wikipedia.org/wiki/Hydrolase hydrolase] (a serine [http://en.wikipedia.org/wiki/Exopeptidase exopeptidase]), breaking N-terminal Xaa-Pro bonds (though it can alΌso catalyze alanine bonds). DPP IV is the founding member of the DPP-IV and/or structure homologue (DASH) family, who all share this serine protease catalysis of post-proline peptide bonds. <ref> PMID: 16186403</ref> These penultimate [http://en.wikipedia.org/wiki/Proline prolines] of the [http://en.wikipedia.org/wiki/N-terminus N-terminus] are known for their ability to resist attacks from most proteases and also induce a [http://en.wikipedia.org/wiki/Conformational_change conformational change] of their respective proteins. Also, DPP IV serves as a binding glycoprotein on the membrane of cells, binding ligands such as [http://en.wikipedia.org/wiki/Adenosine_deaminase adenosine deaminase] with high affinity.<ref name="Gorrell"/> Though this interaction has no known significance as of yet, DPP IV and its ability to catalyze N-terminal prolines gives it a unique specificity and target for pharmaceutical companies to take advantage of. <ref name="regpeps">PMID: 10588446</ref> |
---- | ---- | ||
Revision as of 17:48, 31 March 2014
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
Contents |
Dipeptidyl Peptidase IV
Introduction
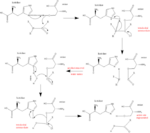
Dipeptidyl Peptidase IV (commonly abbreviated as DPP IV) is a regulatory protease and binding glycoprotein that carries out numerous functions in humans making it a prime candidate for medicinal and pharmaceutical research. DPP IV, discovered by V.K. Hopsu-Havu and G.G. Glenner in homogenized rat liver tissue[1], was originally believed to serve a specific role in breaking 2-Naphthylamine off of Gly-Pro-2-napthylamide, hence its original name glycylproline napthylamidase. However, further research into the specificity of DPP IV eventually showed that it serves a more generic function as a hydrolase (a serine exopeptidase), breaking N-terminal Xaa-Pro bonds (though it can alΌso catalyze alanine bonds). DPP IV is the founding member of the DPP-IV and/or structure homologue (DASH) family, who all share this serine protease catalysis of post-proline peptide bonds. [2] These penultimate prolines of the N-terminus are known for their ability to resist attacks from most proteases and also induce a conformational change of their respective proteins. Also, DPP IV serves as a binding glycoprotein on the membrane of cells, binding ligands such as adenosine deaminase with high affinity.[3] Though this interaction has no known significance as of yet, DPP IV and its ability to catalyze N-terminal prolines gives it a unique specificity and target for pharmaceutical companies to take advantage of. [1]
Structure
| |||||||||||
Medical Relevancy
References
- ↑ 1.0 1.1 Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999 Nov 30;85(1):9-24. PMID:10588446
- ↑ Lankas GR, Leiting B, Roy RS, Eiermann GJ, Beconi MG, Biftu T, Chan CC, Edmondson S, Feeney WP, He H, Ippolito DE, Kim D, Lyons KA, Ok HO, Patel RA, Petrov AN, Pryor KA, Qian X, Reigle L, Woods A, Wu JK, Zaller D, Zhang X, Zhu L, Weber AE, Thornberry NA. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005 Oct;54(10):2988-94. PMID:16186403
- ↑ 3.0 3.1 3.2 3.3 3.4 Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond). 2005 Apr;108(4):277-92. PMID:15584901 doi:http://dx.doi.org/10.1042/CS20040302