We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 914
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
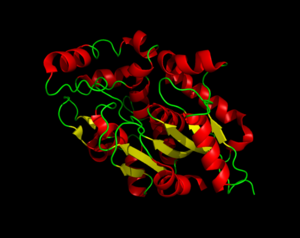
=== α/β Hydrolase Fold === | === α/β Hydrolase Fold === | ||
| - | The α/β Hydrolase Fold is common to many other hydrolases. This fold consists of <scene name='57/573128/4/1'>β3-β8</scene> and αA, αB, αC, and αF. The α/β hydrolase fold has a central 6 stranded parallel Beta sheet. There is also a helix that is roughly perpendicular to the direction of the beta sheet. There is also a large insertion, containing 6 helices, that forms a second domain that encompasses most of the fatty acid binding site. | + | The α/β Hydrolase Fold is common to many other hydrolases. This fold consists of <scene name='57/573128/4/1'>β3-β8</scene> and <scene name='57/573128/5/1'>αA, αB, αC, and αF</scene>. The α/β hydrolase fold has a central 6 stranded parallel Beta sheet. There is also a helix that is roughly perpendicular to the direction of the beta sheet. There is also a large insertion, containing 6 helices, that forms a second domain that encompasses most of the fatty acid binding site. |
=== Catalytic Triad === | === Catalytic Triad === | ||
Revision as of 00:42, 1 April 2014
ββ
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
Palmitoyl-Protein Thioesterase 1
| |||||||||||