Sandbox reserved 915
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
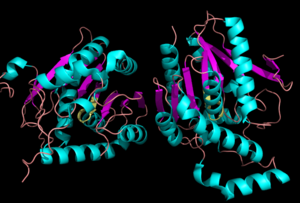
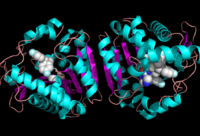
[[Image:Complete_crystal_structure.png|left|300px|thumb|'''Figure 1:'''Crystal Structure of MGL Alpha helixes are in blue and beta sheets in purple. This protein is a dimer that is linked by antiparallel beta sheets]] | [[Image:Complete_crystal_structure.png|left|300px|thumb|'''Figure 1:'''Crystal Structure of MGL Alpha helixes are in blue and beta sheets in purple. This protein is a dimer that is linked by antiparallel beta sheets]] | ||
==Background== | ==Background== | ||
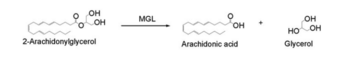
| - | Monoglyceride [[:Category:Lipase| Lipase]] (MGL) is part of the α/β hydrolase family,a [[:Category:Serine hydrolase| Serine hydrolase]]( Figure 1), having a <scene name='58/580298/Catalytic_triad/4'>Ser-His-Asp catalytic triad </scene> <ref name="Clemente">[Clemente, J. C., E. Nulton, M. Nelen, M. J. Todd, D. Maguire, C. Schalk-Hihi, L. C. Kuo, S.-P. Zhang, C. M. Flores, and J. K. Kranz. "Screening and Characterization of Human Monoglyceride Lipase Active Site Inhibitors Using Orthogonal Binding and Functional Assays." Journal of Biomolecular Screening 17.5 (2012): 629-40]</ref>. [http://en.wikipedia.org/wiki/Monoacylglycerol_lipase MGL] is present in most cells, providing the rate limiting step for the hydrolysis of [http://en.wikipedia.org/wiki/Monoglyceride monoacylglycerols] (MG) into fatty acids and glycerol <ref name="Taschler">[Taschler, U., F. P. W. Radner, C. Heier, R. Schreiber, M. Schweiger, G. Schoiswohl, K. Preiss-Landl, D. Jaeger, B. Reiter, H. C. Koefeler, J. Wojciechowski, C. Theussl, J. M. Penninger, A. Lass, G. Haemmerle, R. Zechner, and R. Zimmermann. "Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-induced Insulin Resistance." Journal of Biological Chemistry 286.20 (2011): 17467-7477]</ref> . MGL also terminates the signaling of a primary endocannabinoid, 2-AG <ref name="Savinainen">[Savinainen, Juha R., Megumi Yoshino, Anna Minkkilä, Tapio Nevalainen, and Jarmo T. Laitinen. "Characterization of Binding Properties of Monoglyceride Lipase Inhibitors by a Versatile Fluorescence-based Technique." Analytical Biochemistry 399.1 (2010): 132-34]</ref>. MGL is the main enzyme responsible for hydrolyzing 2-arachidonoylglycerol into arachidonic acid and glycerol ''in vivo'' (Figure 3) <ref name="Bertrand">[ Bertrand, T., F. Augé, J. Houtmann, A. Rak, F. Vallée, V. Mikol, P.f. Berne, N. Michot, D. Cheuret, C. Hoornaert, and M. Mathieu. "Structural Basis for Human Monoglyceride Lipase Inhibition." Journal of Molecular Biology 396.3 (2010): 663-73.]</ref>. One of the key features of MGL is the hydrophobic tunnel, which has been suggested to provide a model for drug research. | + | Monoglyceride [[:Category:Lipase| Lipase]] (MGL) is part of the α/β hydrolase family,a [[:Category:Serine hydrolase| Serine hydrolase]] (Figure 1), having a <scene name='58/580298/Catalytic_triad/4'>Ser-His-Asp catalytic triad </scene> <ref name="Clemente">[Clemente, J. C., E. Nulton, M. Nelen, M. J. Todd, D. Maguire, C. Schalk-Hihi, L. C. Kuo, S.-P. Zhang, C. M. Flores, and J. K. Kranz. "Screening and Characterization of Human Monoglyceride Lipase Active Site Inhibitors Using Orthogonal Binding and Functional Assays." Journal of Biomolecular Screening 17.5 (2012): 629-40]</ref>. [http://en.wikipedia.org/wiki/Monoacylglycerol_lipase MGL] is present in most cells, providing the rate limiting step for the hydrolysis of [http://en.wikipedia.org/wiki/Monoglyceride monoacylglycerols] (MG) into fatty acids and glycerol <ref name="Taschler">[Taschler, U., F. P. W. Radner, C. Heier, R. Schreiber, M. Schweiger, G. Schoiswohl, K. Preiss-Landl, D. Jaeger, B. Reiter, H. C. Koefeler, J. Wojciechowski, C. Theussl, J. M. Penninger, A. Lass, G. Haemmerle, R. Zechner, and R. Zimmermann. "Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-induced Insulin Resistance." Journal of Biological Chemistry 286.20 (2011): 17467-7477]</ref> . MGL also terminates the signaling of a primary endocannabinoid, 2-AG <ref name="Savinainen">[Savinainen, Juha R., Megumi Yoshino, Anna Minkkilä, Tapio Nevalainen, and Jarmo T. Laitinen. "Characterization of Binding Properties of Monoglyceride Lipase Inhibitors by a Versatile Fluorescence-based Technique." Analytical Biochemistry 399.1 (2010): 132-34]</ref>. MGL is the main enzyme responsible for hydrolyzing 2-arachidonoylglycerol into arachidonic acid and glycerol ''in vivo'' (Figure 3) <ref name="Bertrand">[ Bertrand, T., F. Augé, J. Houtmann, A. Rak, F. Vallée, V. Mikol, P.f. Berne, N. Michot, D. Cheuret, C. Hoornaert, and M. Mathieu. "Structural Basis for Human Monoglyceride Lipase Inhibition." Journal of Molecular Biology 396.3 (2010): 663-73.]</ref>. One of the key features of MGL is the hydrophobic tunnel, which has been suggested to provide a model for drug research. |
===Metabolic Role=== | ===Metabolic Role=== | ||
| Line 49: | Line 49: | ||
==Ligand Binding Site== | ==Ligand Binding Site== | ||
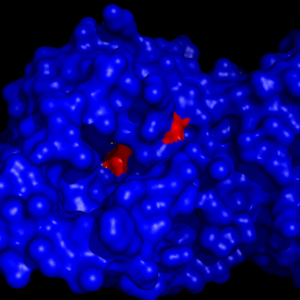
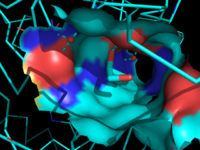
| - | [[Image:Overall_ligand.png|left|200px|thumb|'''Figure 6:''' Ligand within the Overall Structure of MGL]] | + | [[Image:Overall_ligand.png|left|200px|thumb|'''Figure 6:''' Ligand within the Overall Structure of MGL]] [[Image:Ligand_tunnel.png|right|200px|thumb|'''Figure 7:''' Ligand binding pocket showing the hydrophobic and polar regions]] |
The <scene name='58/580298/Ligand/1'>ligand binding pocket</scene> of MGL has a large hydrophobic region with a polar bottom. The entrance of the binding pocket for MGL contains a lid, which is very flexible. The binding pocket or tunnel within MGL matches with the overall structure of 2-AG, with 2-AG's polar head being cleaved by the catalytic triad. Bertrand found that in MGL the binding pocket is not adjusted to the ligand's shape. However, the main movements of MGL associated with ligand binding involved the lid region. When 2-AG and its isomer 1(3)-AG bind to MGL, the hydrophobic chain is first aligned with the left part of the binding pocket. The carbonyl is then hydrogen bonded to <scene name='58/580298/Ala61/1'>Ala61</scene>. The polar head group of the ligand is then fixed by three hydrogen bonds. As a result, future research is looking into the large lipophilic portion of the binding pocket for designing selective inhibitors <ref name="Bertrand" />. | The <scene name='58/580298/Ligand/1'>ligand binding pocket</scene> of MGL has a large hydrophobic region with a polar bottom. The entrance of the binding pocket for MGL contains a lid, which is very flexible. The binding pocket or tunnel within MGL matches with the overall structure of 2-AG, with 2-AG's polar head being cleaved by the catalytic triad. Bertrand found that in MGL the binding pocket is not adjusted to the ligand's shape. However, the main movements of MGL associated with ligand binding involved the lid region. When 2-AG and its isomer 1(3)-AG bind to MGL, the hydrophobic chain is first aligned with the left part of the binding pocket. The carbonyl is then hydrogen bonded to <scene name='58/580298/Ala61/1'>Ala61</scene>. The polar head group of the ligand is then fixed by three hydrogen bonds. As a result, future research is looking into the large lipophilic portion of the binding pocket for designing selective inhibitors <ref name="Bertrand" />. | ||
Revision as of 18:00, 19 April 2014
Monoglyceride Lipase (MGL)
| |||||||||||