We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 919
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
<scene name='58/580297/3dnm_cartoon_dotsribbon/1'>Hormone-sensitive lipases</scene> are generally well-conserved across domains, including prokaryotes, showing 29, 26, and 22% residue overlap in [http://en.wikipedia.org/wiki/Alicyclobacillus ''Alicyclobacillus acidocaldarius''], [http://en.wikipedia.org/wiki/Archaeoglobus ''Archaeoglobus fulgidus''], and [http://en.wikipedia.org/wiki/Bacillus_subtilis ''Bacillus subtilis''], respectively.<ref name="Nam">PMID:19089974</ref> HSL is composed of two main structural domains, consisting of a slightly variable N-terminus (shown in blue in the <scene name='58/580297/3dnm_cartoon/3'>default view</scene>) that is thought to contribute to numerous factors including activity, specificity, regioselectivity, thermophilicity, and thermostability.<ref name="Nam">PMID:19089974</ref> Research speculates that the N-terminal domain, consisting of about 300 residues, mediates protein-protein interactions, and possibly subsequent lipid binding.<ref name= "Yeaman">PMID:14725507</ref> The second domain of HSL is the C-terminal catalytic domain (colors other than blue), which contains serine residue phosphorylation sites as well as the [http://en.wikipedia.org/wiki/Catalytic_triad catalytic triad], viewed <scene name='58/580297/3dnm_triad_zoomedout/1'>here</scene> with ligand β-mercaptoethanol, a charge relay network that is characteristic of many hydrolases, such as [http://proteopedia.org/wiki/index.php/Chymotrypsin chymotrypsin].<ref name= "Yeaman">PMID:14725507</ref> With respect to sequence conservation across species, it has been shown that the catalytic domain, including the triad, is conserved across domains, but the domain containing the N-terminus shows little conservation.<ref name="Nam">PMID:19089974</ref> Size-exclusion chromatography studies have shown that HSL has a <scene name='58/580297/3dnm_cartoon_surface/4'>ligand pocket</scene> that is approximately 16Å deep, suggesting that HSL primarily hydrolyzes shorter chained molecules.<ref name="Nam">PMID:19089974</ref> | <scene name='58/580297/3dnm_cartoon_dotsribbon/1'>Hormone-sensitive lipases</scene> are generally well-conserved across domains, including prokaryotes, showing 29, 26, and 22% residue overlap in [http://en.wikipedia.org/wiki/Alicyclobacillus ''Alicyclobacillus acidocaldarius''], [http://en.wikipedia.org/wiki/Archaeoglobus ''Archaeoglobus fulgidus''], and [http://en.wikipedia.org/wiki/Bacillus_subtilis ''Bacillus subtilis''], respectively.<ref name="Nam">PMID:19089974</ref> HSL is composed of two main structural domains, consisting of a slightly variable N-terminus (shown in blue in the <scene name='58/580297/3dnm_cartoon/3'>default view</scene>) that is thought to contribute to numerous factors including activity, specificity, regioselectivity, thermophilicity, and thermostability.<ref name="Nam">PMID:19089974</ref> Research speculates that the N-terminal domain, consisting of about 300 residues, mediates protein-protein interactions, and possibly subsequent lipid binding.<ref name= "Yeaman">PMID:14725507</ref> The second domain of HSL is the C-terminal catalytic domain (colors other than blue), which contains serine residue phosphorylation sites as well as the [http://en.wikipedia.org/wiki/Catalytic_triad catalytic triad], viewed <scene name='58/580297/3dnm_triad_zoomedout/1'>here</scene> with ligand β-mercaptoethanol, a charge relay network that is characteristic of many hydrolases, such as [http://proteopedia.org/wiki/index.php/Chymotrypsin chymotrypsin].<ref name= "Yeaman">PMID:14725507</ref> With respect to sequence conservation across species, it has been shown that the catalytic domain, including the triad, is conserved across domains, but the domain containing the N-terminus shows little conservation.<ref name="Nam">PMID:19089974</ref> Size-exclusion chromatography studies have shown that HSL has a <scene name='58/580297/3dnm_cartoon_surface/4'>ligand pocket</scene> that is approximately 16Å deep, suggesting that HSL primarily hydrolyzes shorter chained molecules.<ref name="Nam">PMID:19089974</ref> | ||
====Catalytic triad==== | ====Catalytic triad==== | ||
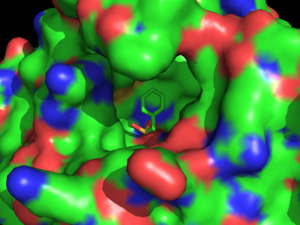
| - | The catalytic triad <scene name='58/580297/3dnm_triad_zoomedin/1'>situates itself</scene> with β-mercaptoethanol toward the middle of HSL. The catalytic triad is composed of residues <scene name='58/580297/3dnm_ligandsite_triad_chains/4'>Ser157, Glu251, and His281</scene>. The Ser157 residue sits at a site deemed the "nucleophilic elbow," that models an approximate torsion of Φ = 60° and Ψ =-120°. This nucleophilic elbow is stabilized by a hydrogen bond between the proximal nitrogen and oxygen atoms of His281 and Glu251, respectively. This model also shows the strong nucleophilic character of Ser157, portraying the interaction and subsequent covalent bonding (not shown) to <scene name='58/580297/3dnm_ligandsite_triad_chains/3'>β-mercaptoethanol</scene>. Return to default view, <scene name='58/580297/3dnm_cartoon/3'>here</scene>. | + | The catalytic triad <scene name='58/580297/3dnm_triad_zoomedin/1'>situates itself</scene> with β-mercaptoethanol toward the middle of HSL. The catalytic triad is composed of residues <scene name='58/580297/3dnm_ligandsite_triad_chains/4'>Ser157, Glu251, and His281</scene>. The Ser157 residue sits at a site deemed the "nucleophilic elbow," that models an approximate torsion of Φ = 60° and Ψ =-120°.<ref name="Nam">PMID:19089974</ref> This nucleophilic elbow is stabilized by a hydrogen bond between the proximal nitrogen and oxygen atoms of His281 and Glu251, respectively. This model also shows the strong nucleophilic character of Ser157, portraying the interaction and subsequent covalent bonding (not shown) to <scene name='58/580297/3dnm_ligandsite_triad_chains/3'>β-mercaptoethanol</scene>. Return to default view, <scene name='58/580297/3dnm_cartoon/3'>here</scene>.<ref name= "Yeaman">PMID:14725507</ref> |
Revision as of 12:41, 22 April 2014
Hormone-sensitive lipase
| |||||||||||
Additional pages about hormone-sensitive lipase
References
- ↑ 1.0 1.1 Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. PMID:14641008 doi:http://dx.doi.org/10.1042/
- ↑ 2.0 2.1 Ray H, Beylot M, Arner P, Larrouy D, Langin D, Holm C, Large V. The presence of a catalytically inactive form of hormone-sensitive lipase is associated with decreased lipolysis in abdominal subcutaneous adipose tissue of obese subjects. Diabetes. 2003 Jun;52(6):1417-22. PMID:12765952
- ↑ 3.0 3.1 3.2 3.3 3.4 Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004 Apr 1;379(Pt 1):11-22. PMID:14725507 doi:http://dx.doi.org/10.1042/BJ20031811
- ↑ 4.0 4.1 4.2 4.3 4.4 Nam KH, Kim MY, Kim SJ, Priyadarshi A, Kwon ST, Koo BS, Yoon SH, Hwang KY. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins. 2009 Mar;74(4):1036-40. PMID:19089974 doi:http://dx.doi.org/10.1002/prot.22313
- ↑ 5.0 5.1 Nam KH, Kim SJ, Priyadarshi A, Kim HS, Hwang KY. The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads. Biochem Biophys Res Commun. 2009 Nov 13;389(2):247-50. Epub 2009 Aug 26. PMID:19715665 doi:10.1016/j.bbrc.2009.08.123
- ↑ Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. Indian J Biochem Biophys. 2005 Aug;42(4):233-7. PMID:23923547
- ↑ James GT. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574-9. PMID:26289
- ↑ Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002 Oct;43(10):1585-94. PMID:12364542