User:Brittany Carroll/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
[[Image:tetra.png|300|left|thumb]] | [[Image:tetra.png|300|left|thumb]] | ||
| - | [[Image:ramachandran.jpg|300|left|thumb]] | ||
== Structural highlights == | == Structural highlights == | ||
| Line 40: | Line 39: | ||
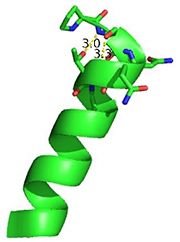
[[Image:Cationpi.png|300|Right|thumb|This image depicts two cation-π interactions between Arg and Tyr or Trp. The energetic significances are -1.22 and -6.55 kj/mol respectively. (site website) (30tb)]] | [[Image:Cationpi.png|300|Right|thumb|This image depicts two cation-π interactions between Arg and Tyr or Trp. The energetic significances are -1.22 and -6.55 kj/mol respectively. (site website) (30tb)]] | ||
A cation-π interaction occurs between a cation and the face of a simple aromatic, there is partial negative charge in the center of the ring. The cation-π interaction is actually stronger than a salt bridge because of the desolvation penalty. With the cation-π interaction the cation has a similar dosolvation penalty to pay as the salt bridge ions but the π system is already poorly solvated. Also there is not neutralization of charge that occurs between the two groups. These properties of the cation-π interaction imply that thecation-π interactions on protein surfaces (mainly where they are seen) could contribute to protein structure and stability. | A cation-π interaction occurs between a cation and the face of a simple aromatic, there is partial negative charge in the center of the ring. The cation-π interaction is actually stronger than a salt bridge because of the desolvation penalty. With the cation-π interaction the cation has a similar dosolvation penalty to pay as the salt bridge ions but the π system is already poorly solvated. Also there is not neutralization of charge that occurs between the two groups. These properties of the cation-π interaction imply that thecation-π interactions on protein surfaces (mainly where they are seen) could contribute to protein structure and stability. | ||
| + | |||
| + | [[Image:ramachandran.jpg|300|left|thumb]] | ||
| + | |||
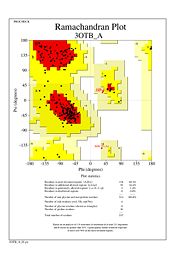
| + | The Ramachandran plot shows that most of the amino acids follow Ramachadran's restraints. The three that are questionable, N32, D67, S75 are all located in turns. | ||
| + | |||
Revision as of 01:30, 27 April 2014
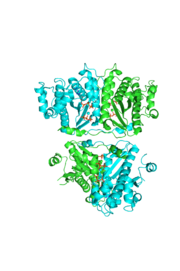
tRNA(His) guanylyltransferase
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644