We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 186
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
Glutathione peroxidase 1 (GPx-1) is a tetramer (23 kDa per monomer) with two units composed of dimers. GPx-1 is the most abundant member of the Glutathione peroxidase family. It is found in all cells and is located in the cytosolic and mitochondrial compartments (1). GPx-1 is a crucial anti-oxidant enzyme that catalyzes the conversion of hydrogen peroxide into water (2). Interestingly GPx-1 contains the rare amino acid selenocysteine which acts as the peroxidatic residue (2). The overall reaction that GPx-1 catalyzes is H2O2 + 2Glutathione (GSH) -> 2H20 + GS-SG (Figure 1). In addition to hydrogen peroxide GPx-1 can reduce other soluble hydroperoxides including lipid hydroperoxides (3). Because of its role in regulating the intracellular concentration of reactive oxygen species, GPx-1 has been found to play a role in numerous processes including cell proliferation, apoptosis, and inflammation (1). Furthermore deficiencies in GPx-1 has been linked to the development of cancers, neurodegenerative diseases, and heart disease (4). | Glutathione peroxidase 1 (GPx-1) is a tetramer (23 kDa per monomer) with two units composed of dimers. GPx-1 is the most abundant member of the Glutathione peroxidase family. It is found in all cells and is located in the cytosolic and mitochondrial compartments (1). GPx-1 is a crucial anti-oxidant enzyme that catalyzes the conversion of hydrogen peroxide into water (2). Interestingly GPx-1 contains the rare amino acid selenocysteine which acts as the peroxidatic residue (2). The overall reaction that GPx-1 catalyzes is H2O2 + 2Glutathione (GSH) -> 2H20 + GS-SG (Figure 1). In addition to hydrogen peroxide GPx-1 can reduce other soluble hydroperoxides including lipid hydroperoxides (3). Because of its role in regulating the intracellular concentration of reactive oxygen species, GPx-1 has been found to play a role in numerous processes including cell proliferation, apoptosis, and inflammation (1). Furthermore deficiencies in GPx-1 has been linked to the development of cancers, neurodegenerative diseases, and heart disease (4). | ||
| - | == Secondary Structure and the Thioredoxin Like Fold == | + | == Secondary Structure and the Thioredoxin Like Fold of GPx-1 == |
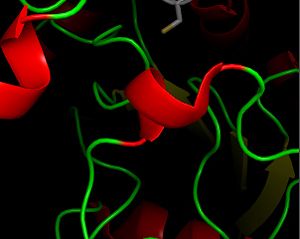
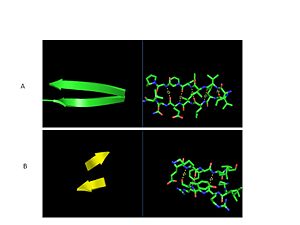
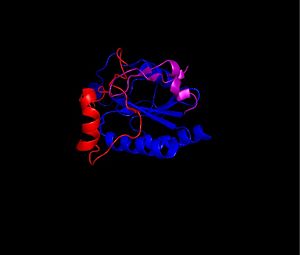
| - | The secondary structure of GPx-1 consists of nine β-strands and nine α-helices with five of the helices being of the 310 form (Figure 2)[[Image:Helix310.jpg|300px|left|thumb|]]. Interestingly two of the β-strands form a parallel β-sheet (Figure 3)[[Image:Beta_sheets3.jpg|300px|right|thumb|]]. GPx-1 exhibits a thioredoxin like fold. The classic thioredoxin fold consists of a four stranded β-sheet that is surrounded by three α-helices (5). However the thioredoxin fold is commonly subject to the insertion of additional secondary structural elements between the second β-strand and the second α-helices (6). This is seen in GPx-1 as there is an addition of an α-helix and a β-strand between the second β-strand and the second α-helices (6). A similar insertion is found in peroxiredoxins, a different family of proteins which also catalyze the reduction of hydroperoxides(6). | + | The secondary structure of GPx-1 consists of nine β-strands and nine α-helices with five of the helices being of the 310 form (Figure 2)[[Image:Helix310.jpg|300px|left|thumb|]]. Interestingly two of the β-strands form a parallel β-sheet (Figure 3)[[Image:Beta_sheets3.jpg|300px|right|thumb|]]. GPx-1 exhibits a thioredoxin like fold. The classic thioredoxin fold consists of a four stranded β-sheet that is surrounded by three α-helices (Figure 4) (5). However the thioredoxin fold is commonly subject to the insertion of additional secondary structural elements between the second β-strand and the second α-helices (6). This is seen in GPx-1 as there is an addition of an α-helix and a β-strand between the second β-strand and the second α-helices (Figure 5)[[Image:Thioredoxin_like_fold.jpg|300px|right|thumb|]] (6). A similar insertion is found in peroxiredoxins, a different family of proteins which also catalyze the reduction of hydroperoxides(6). |
== Relevance == | == Relevance == | ||
Revision as of 06:47, 30 April 2014
| |||||||||||