Hormone sensitive lipase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | __NOTOC__ | |
| + | =Hormone-sensitive lipase= | ||
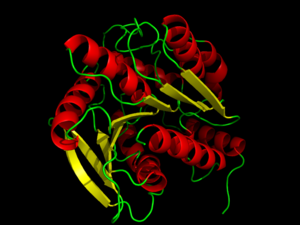
<StructureSection load='3DNM' size='450' side='right' caption='Hormone-Sensitive Lipase (PDB: [[3DNM]])' scene='58/580297/3dnm_cartoon/2' > | <StructureSection load='3DNM' size='450' side='right' caption='Hormone-Sensitive Lipase (PDB: [[3DNM]])' scene='58/580297/3dnm_cartoon/2' > | ||
| Line 19: | Line 20: | ||
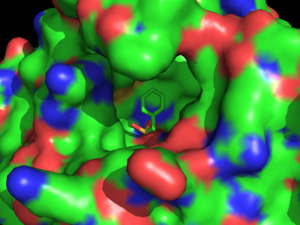
[[Image:SURFACEINHIBITOR.png|300 px|left|thumb|Surface image of Hormone-Sensitive Lipase Complex with PMSF from [http://proteopedia.org/wiki/index.php/3h17 3h17]. Green indicates carbon atoms, blue indicates nitrogen atoms, red indicates oxygen atoms, and orange indicates sulfur atoms.]] | [[Image:SURFACEINHIBITOR.png|300 px|left|thumb|Surface image of Hormone-Sensitive Lipase Complex with PMSF from [http://proteopedia.org/wiki/index.php/3h17 3h17]. Green indicates carbon atoms, blue indicates nitrogen atoms, red indicates oxygen atoms, and orange indicates sulfur atoms.]] | ||
| - | Hormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride ([http://en.wikipedia.org/wiki/PMSF PMSF]) covalently bound to the <scene name='58/580297/Pmsf_surface/4'>active site</scene>. PMSF is a general, covalent inhibitor of | + | Hormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride ([http://en.wikipedia.org/wiki/PMSF PMSF]) covalently bound to the <scene name='58/580297/Pmsf_surface/4'>active site</scene>. PMSF is a general, covalent inhibitor of serine hydrolases, which has been co-crystallized bound to hormone-sensitive lipases. The experiments performed to test this inhibition used different lipases obtained from [http://en.wikipedia.org/wiki/Bacillus_coagulans ''Bacillus coagulans''] as well as lipases from different areas of the human body.<ref name="Kanwar">PMID:23923547</ref> PMSF inhibits hydrolase by binding to the catalytic serine residue of the [http://proteopedia.org/wiki/index.php/Serine_Proteases serine protease] active site which disrupts the nucleophilic activity of the catalytic serine. The sulfur of PMSF binds to the oxygen of the hydroxyl group on the serine residue to form this covalent bond. This inhibitor will only bind to the active site of the catalytic serine because of its participation in the charge relay of the catalytic triad. This hyper activity, indicated by increased temperature (shown in red) around the active site <scene name='58/580296/Meshligand/3'>here</scene>, allows the sulfonyl group of PMSF to covalently bind to the catalytic serine residue to disrupt its activity.<ref name="Kim">PMID: 19715665 </ref> Because of this catalytic serine residue specificity, PMSF does not inhibit all kinds of lipases, such as [http://en.wikipedia.org/wiki/Pancreatic_lipase pancreatic lipase] and lipolase.<ref name="Kanwar"> PMSF is highly degradable in aqueous solutions as it has a [http://en.wikipedia.org/wiki/Half-life half-life] range of 35-110 minutes at [http://en.wikipedia.org/wiki/PH pH] levels of 7.0-8.0.<ref name="Gordon">PMID:26289</ref> <scene name='58/580297/Pmsf_binding/2'>PMSF binding</scene> induces only a minor conformational change from the <scene name='58/580297/3dnm_ligandsite_triad_chains/7'>native protein</scene>.<ref name="Kim"/> |
Increased activity of HSL is also linked to disorders such as atherosclerosis, obesity, and [http://en.wikipedia.org/wiki/Diabetes_mellitus#Type_2 type 2 diabetes]. High concentration of free fatty acids ([http://en.wikipedia.org/wiki/Fatty_acid#Free_fatty_acids FFA]) in skeletal muscles has been reported in many cases of obesity and type 2 diabetes. Increased inhibition of HSL through synthetic inhibitors could be a possible route for decreasing FFA concentration.<ref name="Kraemer">PMID: 12364542 </ref> | Increased activity of HSL is also linked to disorders such as atherosclerosis, obesity, and [http://en.wikipedia.org/wiki/Diabetes_mellitus#Type_2 type 2 diabetes]. High concentration of free fatty acids ([http://en.wikipedia.org/wiki/Fatty_acid#Free_fatty_acids FFA]) in skeletal muscles has been reported in many cases of obesity and type 2 diabetes. Increased inhibition of HSL through synthetic inhibitors could be a possible route for decreasing FFA concentration.<ref name="Kraemer">PMID: 12364542 </ref> | ||
Revision as of 18:20, 3 May 2014
Hormone-sensitive lipase
| |||||||||||
Proteopedia Pages
References
- ↑ 1.0 1.1 Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. PMID:14641008 doi:http://dx.doi.org/10.1042/
- ↑ 2.0 2.1 Ray H, Beylot M, Arner P, Larrouy D, Langin D, Holm C, Large V. The presence of a catalytically inactive form of hormone-sensitive lipase is associated with decreased lipolysis in abdominal subcutaneous adipose tissue of obese subjects. Diabetes. 2003 Jun;52(6):1417-22. PMID:12765952
- ↑ 3.0 3.1 3.2 3.3 3.4 Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004 Apr 1;379(Pt 1):11-22. PMID:14725507 doi:http://dx.doi.org/10.1042/BJ20031811
- ↑ 4.0 4.1 4.2 4.3 4.4 Nam KH, Kim MY, Kim SJ, Priyadarshi A, Kwon ST, Koo BS, Yoon SH, Hwang KY. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins. 2009 Mar;74(4):1036-40. PMID:19089974 doi:http://dx.doi.org/10.1002/prot.22313
- ↑ 5.0 5.1 Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. Indian J Biochem Biophys. 2005 Aug;42(4):233-7. PMID:23923547
- ↑ 6.0 6.1 Nam KH, Kim SJ, Priyadarshi A, Kim HS, Hwang KY. The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads. Biochem Biophys Res Commun. 2009 Nov 13;389(2):247-50. Epub 2009 Aug 26. PMID:19715665 doi:10.1016/j.bbrc.2009.08.123
- ↑ Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002 Oct;43(10):1585-94. PMID:12364542
Student Contributors
Nathan Holt
Derek O'Connor
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, R. Jeremy Johnson, Angel Herraez, Michal Harel