User:Yunlong Zhao/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure features and activation of latent antithrombin == | == Structure features and activation of latent antithrombin == | ||
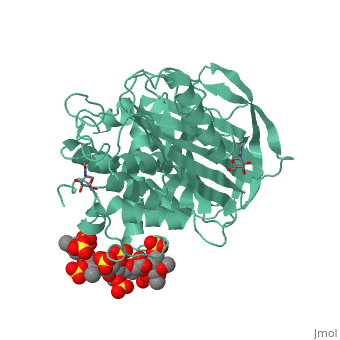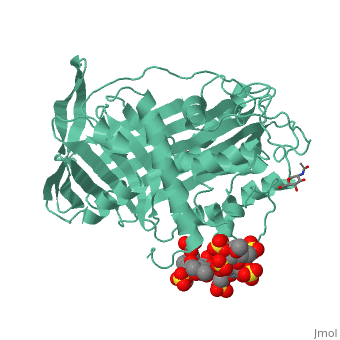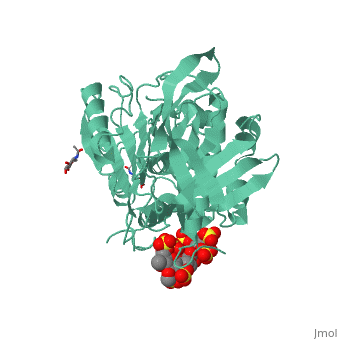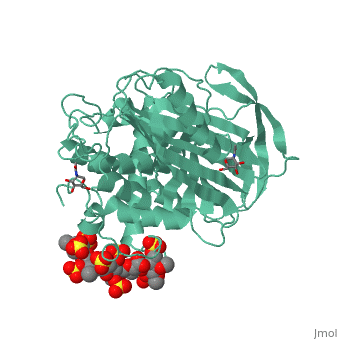
| - | Antithrombin is a natural inhibitor of many serine proteases and it contains an exposed inhibitory loop to intrude the catalytic site of proteases. The peptide bond between R393-S394 plays a critical role in the inhibitory binding (Figure 1). However, the exposure of the inhibitory loop requires a conformational change during activation. In the latent state of antithrombin, this loop is fully buried in the four-strands beta sheet to form a new five-strands beta sheet. This remarkable conformational change of inhibitory loop highlights the activation mechanism of latent antithrombin. | + | Antithrombin is a natural inhibitor of many serine proteases and it contains an exposed inhibitory loop to intrude the catalytic site of proteases. The peptide bond between R393-S394 plays a critical role in the inhibitory binding (Figure 1). However, the exposure of the inhibitory loop requires a conformational change during activation. In the latent state of antithrombin, this loop is fully buried in the four-strands beta sheet to form a new five-strands beta sheet. This remarkable conformational change of inhibitory loop highlights the activation mechanism of latent antithrombin. |
== The mechanism of heparin regulating antithrombin thrombin interaction == | == The mechanism of heparin regulating antithrombin thrombin interaction == | ||
Revision as of 20:59, 6 May 2014
Activation of antithrombin by heparin
| |||||||||||
References
- ↑ Lane DA, Denton J, Flynn AM, Thunberg L, Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725-32. PMID:6721831
- ↑ Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992 Jun 25;267(18):12528-38. PMID:1618758