We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Yunlong Zhao/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
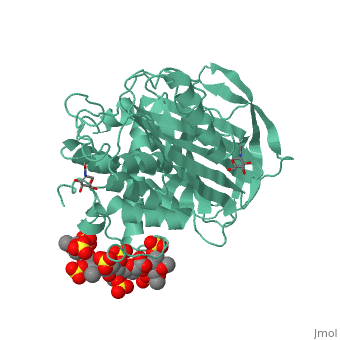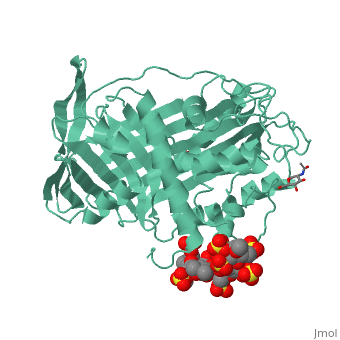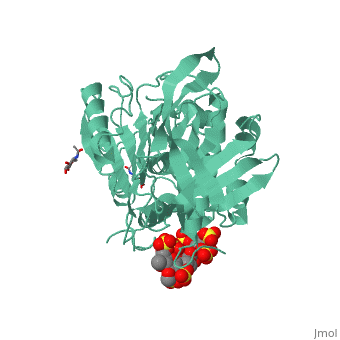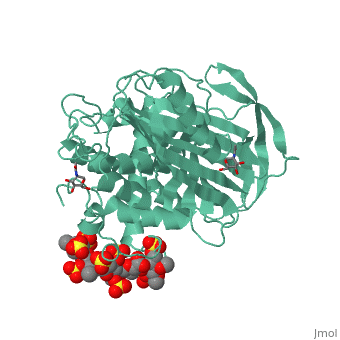
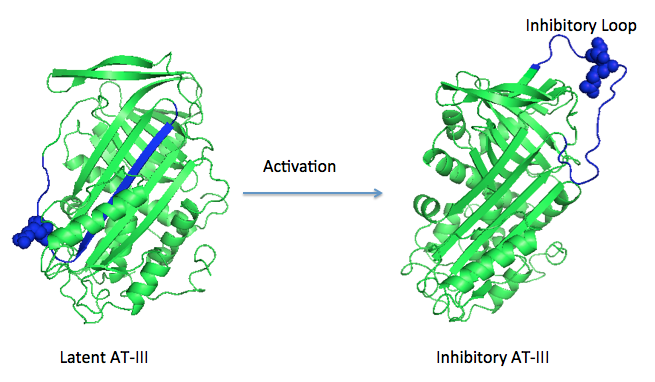
Antithrombin is a natural inhibitor of many serine proteases and it contains an exposed inhibitory loop (shown in blue color in figure 1) to intrude the catalytic site of proteases. The peptide bond between R393-S394 (shown as the sphere model in figure 1) plays a critical role in the inhibitory binding. However, the exposure of the inhibitory loop requires a conformational change during activation. In the latent state of antithrombin, this loop is fully buried in the four-strands beta sheet to form a new five-strands beta sheet. This remarkable conformational change of inhibitory loop highlights the activation mechanism of latent antithrombin, illustrated in fugure 1. | Antithrombin is a natural inhibitor of many serine proteases and it contains an exposed inhibitory loop (shown in blue color in figure 1) to intrude the catalytic site of proteases. The peptide bond between R393-S394 (shown as the sphere model in figure 1) plays a critical role in the inhibitory binding. However, the exposure of the inhibitory loop requires a conformational change during activation. In the latent state of antithrombin, this loop is fully buried in the four-strands beta sheet to form a new five-strands beta sheet. This remarkable conformational change of inhibitory loop highlights the activation mechanism of latent antithrombin, illustrated in fugure 1. | ||
| - | [[Image:Activation_of_AT-III.png]]Figure 1 | + | [[Image:Activation_of_AT-III.png]] |
| + | '''Figure 1''' | ||
== The mechanism of heparin regulating antithrombin thrombin interaction == | == The mechanism of heparin regulating antithrombin thrombin interaction == | ||
Revision as of 04:37, 11 May 2014
Anticoagulation effects of heparin and the interaction with antithrombin
| |||||||||||
References
- ↑ Lane DA, Denton J, Flynn AM, Thunberg L, Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725-32. PMID:6721831
- ↑ Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992 Jun 25;267(18):12528-38. PMID:1618758