User:Yunlong Zhao/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
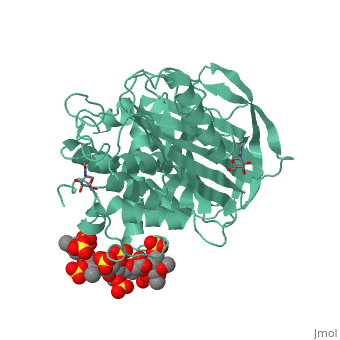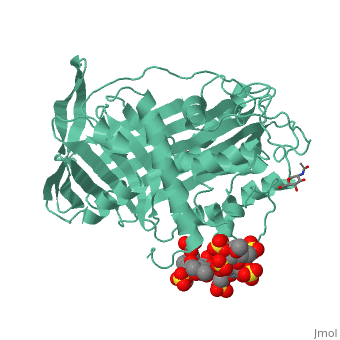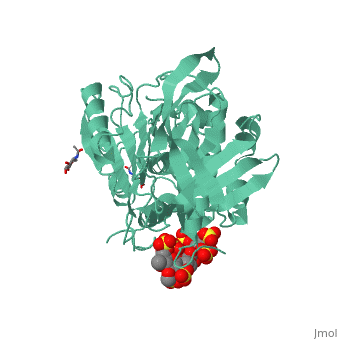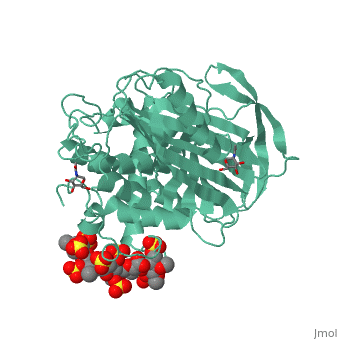
<StructureSection load='1azx' size='340' side='right' caption='Pentasaccharides-bound Antithrombin' scene=''> | <StructureSection load='1azx' size='340' side='right' caption='Pentasaccharides-bound Antithrombin' scene=''> | ||
== Introduction == | == Introduction == | ||
| - | Heparin is a type of glycosaminoglycan chain that is secreted by human mast cell. Heparin exists in many isoforms and is often sulfated at specific sites. Because of the heterogeneity in structure and distribution of negative charge within those sulfate groups, heparin can bind multiple types of proteins and participate in many different biological processes, such as | + | Heparin is a type of glycosaminoglycan chain that is secreted by human mast cell. Heparin exists in many isoforms and is often sulfated at specific sites. Because of the heterogeneity in structure and distribution of negative charge within those sulfate groups, heparin can bind multiple types of proteins and participate in many different biological processes, such as anti-coagulation. Therefore polysaccharides, the low molecular weight products that are originated from digested heparin was often used in the therapy of many diseases such as atrial fibrillation and thrombosis. |
Heparin is usually localized on the surface of cells and the complex with matrix proteins. This interesting discovery suggests that heparin exists in the form of “immobile phase” attaching antithrombin and activates its function as an anticoagulant factor in the circulation system. In an anticoagulant process, antithrombin needs to bind to some coagulant proteases such as Factor Xa or thrombin and “neutralizes” their activity. There are several hypotheses to explain how the heparin chains activate this process. A simple model is that a long heparin chain could be a scaffold to improve the possibility of antithrombin contacting with other proteins. <ref>PMID:6721831</ref> Another direct model is that specific sulfate-containing heparin fragments (oligosaccharides) can allosterically enhance the binding affinity between antithrombin and thrombin or Factor Xa. We are going to describe the structural basis of the second model in the rest of contexts. <ref>PMID:1618758</ref> | Heparin is usually localized on the surface of cells and the complex with matrix proteins. This interesting discovery suggests that heparin exists in the form of “immobile phase” attaching antithrombin and activates its function as an anticoagulant factor in the circulation system. In an anticoagulant process, antithrombin needs to bind to some coagulant proteases such as Factor Xa or thrombin and “neutralizes” their activity. There are several hypotheses to explain how the heparin chains activate this process. A simple model is that a long heparin chain could be a scaffold to improve the possibility of antithrombin contacting with other proteins. <ref>PMID:6721831</ref> Another direct model is that specific sulfate-containing heparin fragments (oligosaccharides) can allosterically enhance the binding affinity between antithrombin and thrombin or Factor Xa. We are going to describe the structural basis of the second model in the rest of contexts. <ref>PMID:1618758</ref> | ||
| Line 13: | Line 13: | ||
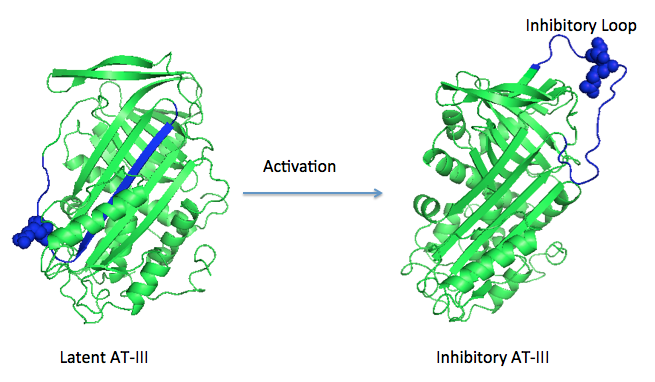
'''Figure 1 The mechanism of latent antithrombin highlighted by a remarkable conformational rearrangement of the inhibitory loop.''' | '''Figure 1 The mechanism of latent antithrombin highlighted by a remarkable conformational rearrangement of the inhibitory loop.''' | ||
| - | == The mechanism of heparin | + | == The allosteric mechanism of heparin-based anticoagulant activation == |
| + | |||
| + | A model of anticoagulant activation of antithrombin by heparin was described as an allosteric mechanism. In another word, heparin chain binds to antithrombin and stabilizes the inhibitory conformation (the inhibitory loop is outward) or alters the antithrombin conformation to facilitate the antithrombin-protease interaction. Early published crystal structure of pentasaccharide-bond antithrombin provided many details of the interaction and validated this hypothesis to some extents. | ||
| - | A model of heparin enhancement of antithrombin-protease interaction was based on an allosteric regulation.Green scene 1 will be the interface between AT and thrombin | ||
Green scene 2 will be the the interface structure plus heparin | Green scene 2 will be the the interface structure plus heparin | ||
Revision as of 04:55, 11 May 2014
Anticoagulation effects of heparin and the interaction with antithrombin
| |||||||||||
References
- ↑ Lane DA, Denton J, Flynn AM, Thunberg L, Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725-32. PMID:6721831
- ↑ Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992 Jun 25;267(18):12528-38. PMID:1618758