Sandbox Reserved 933
From Proteopedia
| Line 19: | Line 19: | ||
The general structure of LEAFY DNA binding domain consists 2 <scene name='57/579703/Beta-strand/1'>β strands</scene> at the beginning followed by 7 <scene name='57/579703/Alpha-helices_color/2'> α helices</scene>. A <scene name='57/579703/Alpha-helices_color/3'>helix-turn-helix</scene> (HTH) motif can be found between α2 and α3 helices, which is recruited to the <scene name='57/579703/Major_groove/2'>major groove </scene>of the binding DNA. There are two amino acid at this motif, <scene name='57/579703/Major_groove_asn291/1'>Asn 291</scene> on α2 and <scene name='57/579703/Major_groove_asn291/2'>Lys 307</scene> on α3 directly mediate site specific recognition with <scene name='57/579703/Major_groove_detail/1'>two guanines</scene> at the DNA strand. These two recognition sites were further validated by electrophoresis mobility shift assay (EMSA): mutation at either Asn 291 or Lys 307 dramatically decrease binding affinity to pAP1. In the minor groove, site specific recognition is conducted by <scene name='57/579703/Arg_237/1'>Arg 237</scene>, which is at the beginning of this structure. ''Arabidopsis'' intermediate mutant ''lfy-4'' (P240L) and ''lfy-5'' (T244M) were located near this site and validate the function ''in planta''. The detail site specific recognition is summaries at right figure produced by PyMol. | The general structure of LEAFY DNA binding domain consists 2 <scene name='57/579703/Beta-strand/1'>β strands</scene> at the beginning followed by 7 <scene name='57/579703/Alpha-helices_color/2'> α helices</scene>. A <scene name='57/579703/Alpha-helices_color/3'>helix-turn-helix</scene> (HTH) motif can be found between α2 and α3 helices, which is recruited to the <scene name='57/579703/Major_groove/2'>major groove </scene>of the binding DNA. There are two amino acid at this motif, <scene name='57/579703/Major_groove_asn291/1'>Asn 291</scene> on α2 and <scene name='57/579703/Major_groove_asn291/2'>Lys 307</scene> on α3 directly mediate site specific recognition with <scene name='57/579703/Major_groove_detail/1'>two guanines</scene> at the DNA strand. These two recognition sites were further validated by electrophoresis mobility shift assay (EMSA): mutation at either Asn 291 or Lys 307 dramatically decrease binding affinity to pAP1. In the minor groove, site specific recognition is conducted by <scene name='57/579703/Arg_237/1'>Arg 237</scene>, which is at the beginning of this structure. ''Arabidopsis'' intermediate mutant ''lfy-4'' (P240L) and ''lfy-5'' (T244M) were located near this site and validate the function ''in planta''. The detail site specific recognition is summaries at right figure produced by PyMol. | ||
=== DNA binding required cooperative dimerization === | === DNA binding required cooperative dimerization === | ||
| - | Transcription factors tent to form homodimer or heterodimer to increase the binding specificity and affinity. Experimental evidence indicates a potential LFY dimer on the binding site. Crystal structure proved that LFY can form dimers at both <scene name='57/579703/Ap1-dimer/2'>pAP1</scene> and <scene name='57/579703/2vy2_assembly/2'>pAG</scene> sites. The binding affinity of LFY protein dimer binds increased by 90-fold compared to the first LFY monomer in EMSA assay. Detailed structure revealed that the contact of two dimerized protein is mediated by <scene name='57/579703/Ap1-dimer/8'>three residues</scene> located at on helix (<scene name='57/579703/Ap1-dimer/3'>α7</scene>) and one loop (<scene name='57/579703/Ap1-dimer/4'>loop12</scene>) at the other protein. Hydrogen bonds can be formed between <scene name='57/579703/Ap1-dimer/7'>Asn 290</scene> and <scene name='57/579703/Ap1-dimer/5'>His 387</scene>/<scene name='57/579703/Ap1-dimer/6'>Arg 390</scene> are essential for this dimeriation. Mutation in any of these three amino acids abolished the binding. Recently, another experiment showing that despite these three residues, the entire N-terminal consensus is critical important for stabilizing the homodimerization, where strong physical interaction can be found by GST-pull down, Y2H and BiFC experiment at in vitro, in vivo and in planta level. | + | Transcription factors tent to form homodimer or heterodimer to increase the binding specificity and affinity. Experimental evidence indicates a potential LFY dimer on the binding site. Crystal structure proved that LFY can form dimers at both <scene name='57/579703/Ap1-dimer/2'>pAP1</scene> and <scene name='57/579703/2vy2_assembly/2'>pAG</scene> sites. The binding affinity of LFY protein dimer binds increased by 90-fold compared to the first LFY monomer in EMSA assay. Detailed structure revealed that the contact of two dimerized protein is mediated by <scene name='57/579703/Ap1-dimer/8'>three residues</scene> located at on helix (<scene name='57/579703/Ap1-dimer/3'>α7</scene>) and one loop (<scene name='57/579703/Ap1-dimer/4'>loop12</scene>) at the other protein. Hydrogen bonds can be formed between <scene name='57/579703/Ap1-dimer/7'>Asn 290</scene> and <scene name='57/579703/Ap1-dimer/5'>His 387</scene>/<scene name='57/579703/Ap1-dimer/6'>Arg 390</scene> are essential for this dimeriation. The detailed interaction is shown in the right figure produced by Pymol. Mutation in any of these three amino acids abolished the binding in EMSA assay. Recently, another experiment showing that despite these three residues, the entire N-terminal consensus is critical important for stabilizing the homodimerization, where strong physical interaction can be found by GST-pull down, Y2H and BiFC experiment at in vitro, in vivo and in planta level. |
</StructureSection> | </StructureSection> | ||
== LEAFY Evolution == | == LEAFY Evolution == | ||
Revision as of 06:55, 18 May 2014
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
Evolution of DNA binding domain of LEAFY: from angiosperms to mosses
Introduction
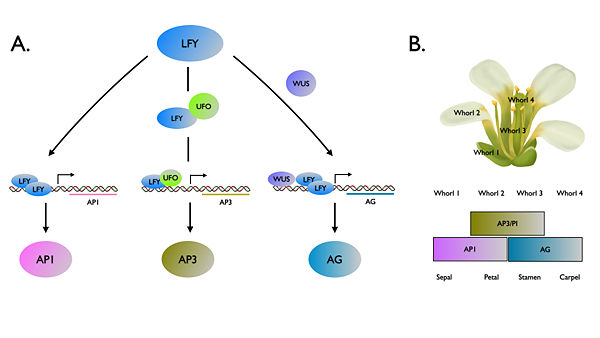
FLORICAULA/LEAFY (FLO/LFY) genes encode a plant specific transcription factor family that controlling floral fate of reproductive phase. [1] In the plant model system Arabidopsis thaliana , ‘’LFY’’ also acts upstream of floral homeotic genes to modulate organ identity. [2] LFY activates the organ identity genes by binding to promoter regions of floral organ identity genes. LFY can directly bind to the promoter to APELATA1 (AP1), while co-regulators UNUSUAL FLORAL ORGANS (UFO) (ref 3) and WUSCHEL (WUS) (ref 4) are required for increment of binding affinity to promoter regions of APELATA3 (AP3) and AGAMOUS (AG), respectively. The exact mechanism how LFY binds to these promoters has yet to be well elucidated until the first structure report about and (ref 5). Among land plants, FLO/LFY homologs share a highly conserved DNA binding region that a hypothesis claimed substitution in this domain might result in the functional divergence (ref 6). Recently, a new study (ref 7) provided new insights of structural basis of LEAFY evolution by changing DNA binding activity.
| |||||||||||
LEAFY Evolution
Reference
- ↑ Detlef Weigel, John Alvarez, David R. Smyth, Martin F. Yanofsky, Elliot M. Meyerowitz, LEAFY controls floral meristem identity in Arabidopsis. Cell 69 :843-859, http://dx.doi.org/10.1016/0092-8674(92)90295-N.
- ↑ Irish, V. F. (2010), The flowering of Arabidopsis flower development. The Plant Journal, 61: 1014–1028. http://doi:10.1111/j.1365-313X.2009.04065.x