We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox bcce04
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
=='''Structure'''== | =='''Structure'''== | ||
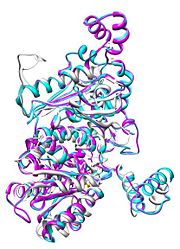
| - | [[Image:Align.jpg|thumb|left|'''Figure 1.''' Multiple alignment IGI - ''Geobacillus stearothermophilus'' (white),'' Homo sapiens'' (pink), ''Oryctolagus cuniculus'' (blue)<ref> | + | [[Image:Align.jpg|thumb|left|'''Figure 1.''' Multiple alignment IGI - ''Geobacillus stearothermophilus'' (white),'' Homo sapiens'' (pink), ''Oryctolagus cuniculus'' (blue)<ref>7</ref>]] |
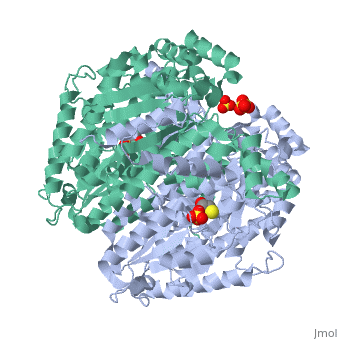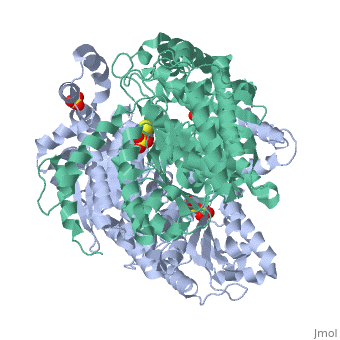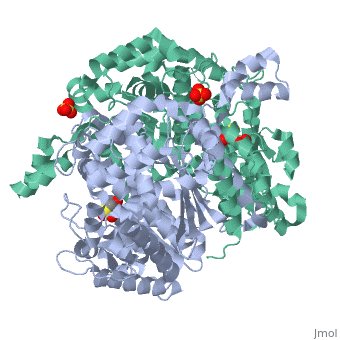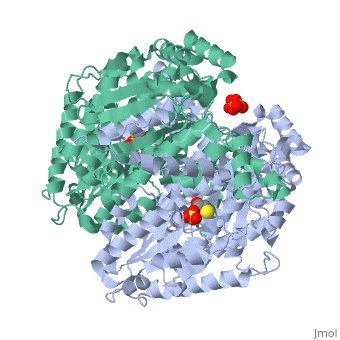
Imaginary isomerase exists in the cell usually as a <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Dimer/1'>homodimer</scene>, nevertheless outside of the cell, it has been isolated as a <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Monomer/1'>monomeric</scene> structure. IGI has essentially an identical fold in all of the characterized species (see '''Figure 1'''). The <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Sec_struct/1'>secondary structure</scene> of imaginary isomerase is charaterized by an αβα conformation, on each of its two domains. The smaller domain is characterized by 5 parallel β-sheets, while the larger domain if formed out of 6 parallel/antiparallel β-sheets. Furthermore, another characteristic trait is a residue extension at the C-terminus, which wraps around the other monomer in the dimeric conformation. A "hook" that can potentially be involved in the previously mentioned extracellular activities. | Imaginary isomerase exists in the cell usually as a <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Dimer/1'>homodimer</scene>, nevertheless outside of the cell, it has been isolated as a <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Monomer/1'>monomeric</scene> structure. IGI has essentially an identical fold in all of the characterized species (see '''Figure 1'''). The <scene name='Stancu_Phosphoglucoisomerase_Sandbox_1/Sec_struct/1'>secondary structure</scene> of imaginary isomerase is charaterized by an αβα conformation, on each of its two domains. The smaller domain is characterized by 5 parallel β-sheets, while the larger domain if formed out of 6 parallel/antiparallel β-sheets. Furthermore, another characteristic trait is a residue extension at the C-terminus, which wraps around the other monomer in the dimeric conformation. A "hook" that can potentially be involved in the previously mentioned extracellular activities. | ||
Revision as of 14:29, 6 August 2014
Imaginary Protein
| |||||||||||
Mechanism
Regulation and Inhibition
3D structures of phosphoglucose isomerase
IGI
2pgi, 1b0z – GsPGI – Geobacillus stearothermophilus
PGI complex with imaginary-6-phosphate
1hox – rPGI + fructose-6-phosphate
PGI complex with pseudoreality-6-phosphate
1xtb - rPGI + sorbitol-6-phosphate
PGI complex with imaginary-6-phosphate
1u0f - mPGI + glucose-6-phosphate