This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Quinone reductase
From Proteopedia
| Line 14: | Line 14: | ||
{{Clear}} | {{Clear}} | ||
| - | |||
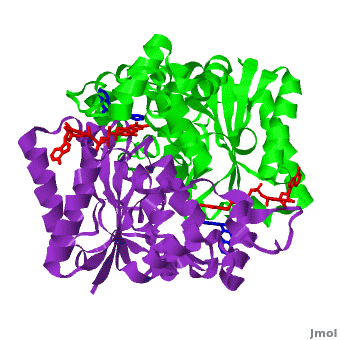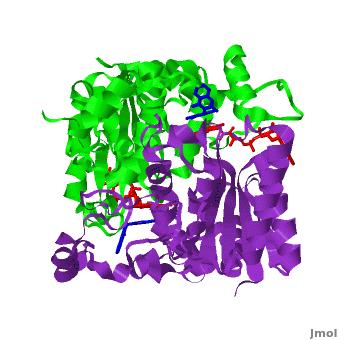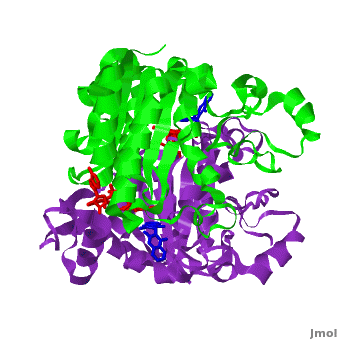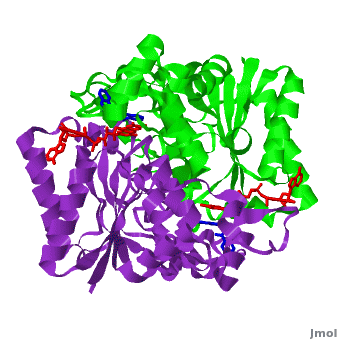
<scene name='2f1o/Align/8'>Structural comparison</scene> of the active site of <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font> (residues important for ligand interactions are <font color='magenta'><b>colored magenta</b></font>) with that of <font color='blue'><b>apo hNQO1</b></font> dimer ([[1d4a]], residues important for ligand interactions are <font color='blue'><b>colored blue</b></font>) reveals that structural changes associated with dicoumarol binding occur on several residues involving both monomers. <font color='cyan'><b>Dicoumarol is colored in cyan</b></font>; <font color='orange'><b>FAD is colored in orange</b></font>. The RMSD between the apo hNQO1 ([[1d4a]]) and hNQO1 in complex with dicoumarol is 0.36Å for the 546 Cα atoms. The dicoumarol-hNQO1 binding causes several structural changes. The most prominent of them is Tyr 128 and Phe 232 movement in the first monomer. These residues are located on the surface of the NQO1 catalytic pocket. The <scene name='2f1o/Align/9'>distance</scene> between these residues increases from ~5 Å in the <font color='blue'><b>apo hNQO1</b></font> to ~12 Å in the <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font>. | <scene name='2f1o/Align/8'>Structural comparison</scene> of the active site of <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font> (residues important for ligand interactions are <font color='magenta'><b>colored magenta</b></font>) with that of <font color='blue'><b>apo hNQO1</b></font> dimer ([[1d4a]], residues important for ligand interactions are <font color='blue'><b>colored blue</b></font>) reveals that structural changes associated with dicoumarol binding occur on several residues involving both monomers. <font color='cyan'><b>Dicoumarol is colored in cyan</b></font>; <font color='orange'><b>FAD is colored in orange</b></font>. The RMSD between the apo hNQO1 ([[1d4a]]) and hNQO1 in complex with dicoumarol is 0.36Å for the 546 Cα atoms. The dicoumarol-hNQO1 binding causes several structural changes. The most prominent of them is Tyr 128 and Phe 232 movement in the first monomer. These residues are located on the surface of the NQO1 catalytic pocket. The <scene name='2f1o/Align/9'>distance</scene> between these residues increases from ~5 Å in the <font color='blue'><b>apo hNQO1</b></font> to ~12 Å in the <font color='magenta'><b>dicoumarol/hNQO1 complex</b></font>. | ||
Quinones (including duroquinone (2,3,5,6-tetramethyl-''p''-benzoquinone) are substrates of NQO1 (it catalyzes two-electron reduction of them to hydroquinones). Duroquinone <font color='black'><b>(yellow)</b></font> binds to the <scene name='2f1o/Align1/4'>active site</scene> by interactions involving the FAD and several hydrophobic and hydrophilic residues in the duroquinone-NQO1 complex ([[1dxo]]). The structure of the hNQO1 dimer in complex with duroquinone is also similar to that of hNQO1 in complex with dicoumarol (RMSD is 0.33Å for the 546 Cα atoms). In this case, the main differences between these two structures, as well as to that of apo hNQO1, involve the distance between residues <scene name='2f1o/Align1/5'>Tyr 128 and Phe 232</scene> of the first monomer. The FAD molecule has very similar conformation in both hNQO1-duroquinone <font color='pink'><b>(pink)</b></font> and hNQO1−dicoumarol <font color='orange'><b>(orange)</b></font> complexes. Based on the comparison of NQO1 structure in complex with different NQO1 inhibitors and our previous analysis of NQO1 mutations that affect NQO1 interactions we propose that the specific conformation of Tyr 128 and Phe 232 is important for NQO1 interaction with p53 and other client proteins. | Quinones (including duroquinone (2,3,5,6-tetramethyl-''p''-benzoquinone) are substrates of NQO1 (it catalyzes two-electron reduction of them to hydroquinones). Duroquinone <font color='black'><b>(yellow)</b></font> binds to the <scene name='2f1o/Align1/4'>active site</scene> by interactions involving the FAD and several hydrophobic and hydrophilic residues in the duroquinone-NQO1 complex ([[1dxo]]). The structure of the hNQO1 dimer in complex with duroquinone is also similar to that of hNQO1 in complex with dicoumarol (RMSD is 0.33Å for the 546 Cα atoms). In this case, the main differences between these two structures, as well as to that of apo hNQO1, involve the distance between residues <scene name='2f1o/Align1/5'>Tyr 128 and Phe 232</scene> of the first monomer. The FAD molecule has very similar conformation in both hNQO1-duroquinone <font color='pink'><b>(pink)</b></font> and hNQO1−dicoumarol <font color='orange'><b>(orange)</b></font> complexes. Based on the comparison of NQO1 structure in complex with different NQO1 inhibitors and our previous analysis of NQO1 mutations that affect NQO1 interactions we propose that the specific conformation of Tyr 128 and Phe 232 is important for NQO1 interaction with p53 and other client proteins. | ||
| Line 21: | Line 20: | ||
The quinone ES936 causes irreversible inhibition of the NQO1. <scene name='2f1o/Align2/5'>Alignment</scene> of the hNQO1 dimer in complex with <font color='red'><b>ES936 (red)</b></font> ([[1kbq]]) with the hNQO1−dicoumarol complex ([[2f1o]]) yields 0.45Å RMSD for the 546 Cα atoms. The ES936 causes structural change only in the position of Phe 232. The movement of this residue is smaller than that caused by dicoumarol. The <scene name='2f1o/Align2/6'>distance</scene> between Tyr 128 and Phe 232 in the hNQO1−ES936 complex is only ~7 Å, while in the hNQO1−dicoumarol complex it is ~12 Å. | The quinone ES936 causes irreversible inhibition of the NQO1. <scene name='2f1o/Align2/5'>Alignment</scene> of the hNQO1 dimer in complex with <font color='red'><b>ES936 (red)</b></font> ([[1kbq]]) with the hNQO1−dicoumarol complex ([[2f1o]]) yields 0.45Å RMSD for the 546 Cα atoms. The ES936 causes structural change only in the position of Phe 232. The movement of this residue is smaller than that caused by dicoumarol. The <scene name='2f1o/Align2/6'>distance</scene> between Tyr 128 and Phe 232 in the hNQO1−ES936 complex is only ~7 Å, while in the hNQO1−dicoumarol complex it is ~12 Å. | ||
</StructureSection> | </StructureSection> | ||
| - | + | ||
== 3D Structures of Quinone reductase == | == 3D Structures of Quinone reductase == | ||
Revision as of 07:56, 20 August 2014
| |||||||||||
Contents |
3D Structures of Quinone reductase
Updated on 20-August-2014
Quinone reductase type 1
3jsx – hQR1 + coumarine derivative
2f1o – hQR1 + dicoumarol
1kbo, 1kbq – hQR1 + indole derivative
Quinone reductase type 2
3fw1, 1qr2 – hQR2 - human
3o2n, 3g5m, 3gam – hQR2 + PET agent
3ovm, 3owh, 3owx – hQR2 + carbamate derivative
3ox1, 2qx4, 2qx6, 2qx8, 2qx9, 2qwx – hQR2 + acetamide derivative
3ox2 - hQR2 + indole derivative
3ox3 - hQR2 + carboxamide derivative
2qmy – hQR2 + adrenochrome
2qr2 – hQR2 + menadione
2qmz – hQR2 + dopamine
1sg0 – hQR2 + resveratol
3nhu, 3nhs, 3nhr, 3nhp, 3nhl, 3nhk, 3nhj, 3nhf, 3nfr, 3nhw, 3nhy, 3uxe, 3uxh - hQR2 + quinoline derivative
3te7, 3tem, 3tzb – hQR2 + acridine derivative
2bzs, 1xi2, 1zx1, 3o73 – hQR2 + anti-cancer prodrug
4fgj, 4fgk, 4fgl – hQR2 + anti-malaria drug
4gqi, 4gr9 – hQR2 + ligand
Sulfide-quinone reductase
3hyv – AaSQR – Aquifex aeolicus
3hyw – AaSQR + decylubiquinone
3hyx – AaSQR + Aurachin C
NADPH-quinone reductase
3ha2 – NQR – Pediococcus pentosaceus
1dxq, 1d4a - hNQR
1yb5 – hNQR + NADP
1gg5, 1h66, 1h69, 1qbg - hNQR + anti-cancer prodrug
1dxo - hNQR + quinone derivative
1qrd – QR + bicarbon blue + duroquinone - rat
4gi5 – QR + FAD – Klebsiella pneumoniae
References
- Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3177-82. PMID:10706635 doi:http://dx.doi.org/10.1073/pnas.050585797
- Asher G, Dym O, Tsvetkov P, Adler J, Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006 May 23;45(20):6372-8. PMID:16700548 doi:10.1021/bi0600087