Vascular Endothelial Growth Factor
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | ||
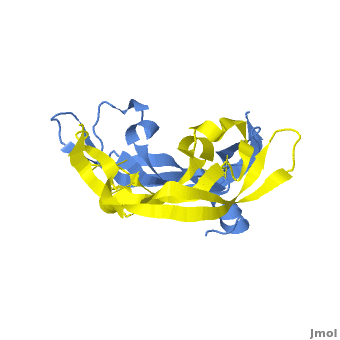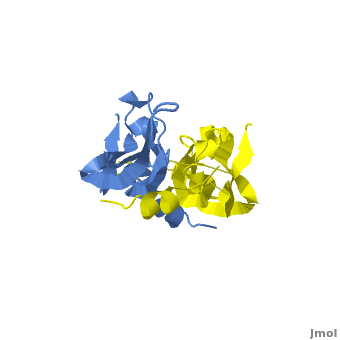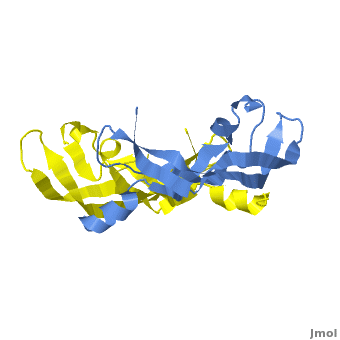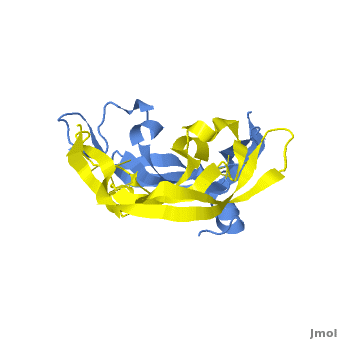
<StructureSection load='1vpf2.pdb' size='400' side='right' scene='Vascular_Endothelial_Growth_Factor/Vegf-a_opening/1' caption='Structure of Human VEGF-A dimer, [[1vpf]]'> | <StructureSection load='1vpf2.pdb' size='400' side='right' scene='Vascular_Endothelial_Growth_Factor/Vegf-a_opening/1' caption='Structure of Human VEGF-A dimer, [[1vpf]]'> | ||
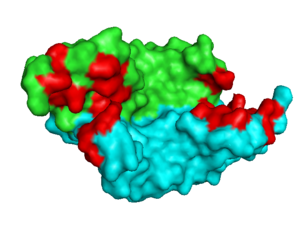
[[Image: VEGF_Opening.png|300px|left|thumb| VEGF-A highlighting 2 monomers and binding sites in red, [[1vpf]]]] | [[Image: VEGF_Opening.png|300px|left|thumb| VEGF-A highlighting 2 monomers and binding sites in red, [[1vpf]]]] | ||
| Line 6: | Line 6: | ||
<br /> | <br /> | ||
| - | __NOTOC__ | ||
<br /> | <br /> | ||
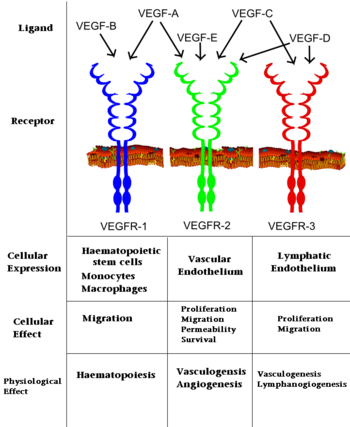
[[Image: VEGF_effects.PNG|350px|left|thumb| Interaction of VEGFs with VEGFRs. ]] | [[Image: VEGF_effects.PNG|350px|left|thumb| Interaction of VEGFs with VEGFRs. ]] | ||
| Line 12: | Line 11: | ||
==History and Biological Function== | ==History and Biological Function== | ||
VEGF-A was first described by Senger ''et al.'' in 1983 as a tumor secreted “vascular-permeability factor (VPF). <ref>PMID:6823562</ref> In 1989, Henzel and Ferrara reported the isolation of an endothelial cell mitogen they named VEGF which also mediated vascular permeability in vivo. Subsequent sequencing revealed that VPF and VEGF were identical, with the VEGF moniker sticking. <ref>PMID: 2735925</ref>. VEGF represents a family of homodimeric glycoprotins which are essential for vasculogenesis (embryonic development of blood vessels), Lymphangiogenesis (lymphatic system development) and angiogenesis (formation of new blood vessels from pre-existing ones). <ref name="Ferrara">PMID:15294883</ref> VEGF-A, arguably the most important member of the VEGF family, belongs to a gene family that includes placenta growth factor (PIGF) and VEGF’s B, C, D, E (Viral), and F (found in snake toxin) <ref>PMID:15542594</ref>. | VEGF-A was first described by Senger ''et al.'' in 1983 as a tumor secreted “vascular-permeability factor (VPF). <ref>PMID:6823562</ref> In 1989, Henzel and Ferrara reported the isolation of an endothelial cell mitogen they named VEGF which also mediated vascular permeability in vivo. Subsequent sequencing revealed that VPF and VEGF were identical, with the VEGF moniker sticking. <ref>PMID: 2735925</ref>. VEGF represents a family of homodimeric glycoprotins which are essential for vasculogenesis (embryonic development of blood vessels), Lymphangiogenesis (lymphatic system development) and angiogenesis (formation of new blood vessels from pre-existing ones). <ref name="Ferrara">PMID:15294883</ref> VEGF-A, arguably the most important member of the VEGF family, belongs to a gene family that includes placenta growth factor (PIGF) and VEGF’s B, C, D, E (Viral), and F (found in snake toxin) <ref>PMID:15542594</ref>. | ||
| - | |||
VEGF is produced by a range of cells including tumor cells, vascular smooth muscle cells, and macrophages. The VEGF-A gene contains a hypoxia responsive element, and hypoxia induces rapid production of VEGF-mRNA<ref>PMID:1279431</ref>.The biological function of VEGFs is predicated upon binding to three receptor tyrosine kinases, VEGFR-1-3, that are typically localized on the surface of endothelial cells, bone marrow derived hematopoetic precursor cells, and some malignant cells. <ref>PMID:9034784</ref> VEGF identity determines which of the VEGFRs it binds with high affinity (See Image at the left). VEGF binding of a VEGFR begins a cascade reaction which ultimately leads to angiogenesis, vasculogenesis or haematopoiesis. The biological importance of VEGFs, especially VEGF-A, is highlighted by the fact that VEGF-A -/- knockout mice exhibit severe defects in vascular development and die at E9.5-10.5 <ref>PMID:8602241</ref>. Additionally, loss of just a single VEGF-A allele leads to vascular defects and death by E11-12 <ref>PMID:8602241</ref>. | VEGF is produced by a range of cells including tumor cells, vascular smooth muscle cells, and macrophages. The VEGF-A gene contains a hypoxia responsive element, and hypoxia induces rapid production of VEGF-mRNA<ref>PMID:1279431</ref>.The biological function of VEGFs is predicated upon binding to three receptor tyrosine kinases, VEGFR-1-3, that are typically localized on the surface of endothelial cells, bone marrow derived hematopoetic precursor cells, and some malignant cells. <ref>PMID:9034784</ref> VEGF identity determines which of the VEGFRs it binds with high affinity (See Image at the left). VEGF binding of a VEGFR begins a cascade reaction which ultimately leads to angiogenesis, vasculogenesis or haematopoiesis. The biological importance of VEGFs, especially VEGF-A, is highlighted by the fact that VEGF-A -/- knockout mice exhibit severe defects in vascular development and die at E9.5-10.5 <ref>PMID:8602241</ref>. Additionally, loss of just a single VEGF-A allele leads to vascular defects and death by E11-12 <ref>PMID:8602241</ref>. | ||
| Line 19: | Line 17: | ||
==Structure of VEGF-A & its Biology== | ==Structure of VEGF-A & its Biology== | ||
<scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_opening/1'>VEGF-A</scene> is a homodimer composed of two 23 kDa subunits. VEGF-A exists in a number of different isoforms following alternative splicing of its precursor mRNA <ref>PMID: 11181169</ref>. In humans, 6 variants have been found: VEGF-A-121, VEGF-A-145, VEGF-A-165, VEGF-A-183, VEGF-A-189, and VEGF-A-206, with VEGF-A-165 the most abundantly expressed. All VEGF-A isoforms bind to VEGFR-1 and -2. | <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_opening/1'>VEGF-A</scene> is a homodimer composed of two 23 kDa subunits. VEGF-A exists in a number of different isoforms following alternative splicing of its precursor mRNA <ref>PMID: 11181169</ref>. In humans, 6 variants have been found: VEGF-A-121, VEGF-A-145, VEGF-A-165, VEGF-A-183, VEGF-A-189, and VEGF-A-206, with VEGF-A-165 the most abundantly expressed. All VEGF-A isoforms bind to VEGFR-1 and -2. | ||
| - | |||
The amino acids determined to be <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_binding_to_vegfr1/2'>critical to binding to VEGFR-1</scene> are D63, L66, and E67. VEGF-A binding by VEGFR-1 leads to cellular proliferation, migration, and increased cellular permeability resulting in vasculogenesis and angiogenesis. Those residues <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_binding_to_vegfr2/2'>critical to binding to VEGFR-2</scene> are I43, I46, Q79, I83, K84 and P85.<ref>pmid:9207067</ref> Binding of VEGF-A to VEGFR-2 results in similar Vasculogenesis and angiogenesis, but also lymphangiogenesis in embryos. The remainder of the <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_full_binding_site/1'>binding pocket </scene> is formed by D34, S50, E64, and F36. It is upon binding of VEGFR by VEGF that the subsequent signal cascade is initiated leading to angiogenesis, etc.<ref>pmid:8621427</ref> | The amino acids determined to be <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_binding_to_vegfr1/2'>critical to binding to VEGFR-1</scene> are D63, L66, and E67. VEGF-A binding by VEGFR-1 leads to cellular proliferation, migration, and increased cellular permeability resulting in vasculogenesis and angiogenesis. Those residues <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_binding_to_vegfr2/2'>critical to binding to VEGFR-2</scene> are I43, I46, Q79, I83, K84 and P85.<ref>pmid:9207067</ref> Binding of VEGF-A to VEGFR-2 results in similar Vasculogenesis and angiogenesis, but also lymphangiogenesis in embryos. The remainder of the <scene name='Vascular_Endothelial_Growth_Factor/Vegf-a_full_binding_site/1'>binding pocket </scene> is formed by D34, S50, E64, and F36. It is upon binding of VEGFR by VEGF that the subsequent signal cascade is initiated leading to angiogenesis, etc.<ref>pmid:8621427</ref> | ||
| - | __NOTOC__ | ||
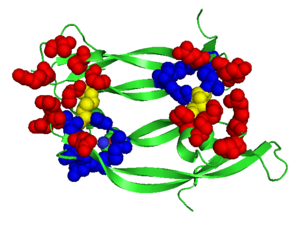
[[Image: VEGFE_Opening.png|300px|left|thumb| Structure of VEGF-E. Red Highlights Binding sites, Blue Highlights the Cysteine Knot, Yellow Highlights the intermolecular Dislufide bonds, [[2gnn]]]] | [[Image: VEGFE_Opening.png|300px|left|thumb| Structure of VEGF-E. Red Highlights Binding sites, Blue Highlights the Cysteine Knot, Yellow Highlights the intermolecular Dislufide bonds, [[2gnn]]]] | ||
{{Clear}} | {{Clear}} | ||
| Line 29: | Line 25: | ||
==Structure of VEGF-E as a Model== | ==Structure of VEGF-E as a Model== | ||
Although VEGF-E is only found in viral sources and thus is of less importance than VEGF-A, analysis of its structure is informative because it is the only member of the VEGF family that binds exclusively to VEGFR-2, the most essential VEGF receptor. Further, VEGF-E shares significant homology to VEGF-A, and thus can serve as an effective model. <ref>PMID:16672228</ref> | Although VEGF-E is only found in viral sources and thus is of less importance than VEGF-A, analysis of its structure is informative because it is the only member of the VEGF family that binds exclusively to VEGFR-2, the most essential VEGF receptor. Further, VEGF-E shares significant homology to VEGF-A, and thus can serve as an effective model. <ref>PMID:16672228</ref> | ||
| - | |||
<scene name='Vascular_Endothelial_Growth_Factor/Vegf-e_opening/1'>VEGF-E</scene> consists of a homodimer that is covalently linked by two intermolecular disulfide bonds between <scene name='Vascular_Endothelial_Growth_Factor/Vegf-e_disulfide_5061/1'>Cys51 and Cys 60</scene>. Each monomer contains a central antiparallel beta sheet, with the canonical | <scene name='Vascular_Endothelial_Growth_Factor/Vegf-e_opening/1'>VEGF-E</scene> consists of a homodimer that is covalently linked by two intermolecular disulfide bonds between <scene name='Vascular_Endothelial_Growth_Factor/Vegf-e_disulfide_5061/1'>Cys51 and Cys 60</scene>. Each monomer contains a central antiparallel beta sheet, with the canonical | ||
Revision as of 09:30, 21 August 2014
| |||||||||||
Contents |
3D Structures of VEGF
Updated on 21-August-2014
VEGF-A
3bdy, 2qr0 - hVEGF-A + hFab light+heavy chain – human
4kzn - hVEGF-A receptor-binding domain
2fjg, 2fjh - hVEGF-A receptor-binding domain+ hFab light+heavy chain
3p9w - hVEGF-A + hFab heavy chain
1tzh, 1tzi, 1cz8 - hVEGF-A + mFab YADS1 light+heavy chain - mouse
1mkg, 1mkk, 1mjv – hVEGF-A (mutant)
3qtk - hVEGF-A residues 34-135
4glu - hVEGF-A residues 1-102
1kmx - hVEGF-A heparin-binding domain
1vgh, 2vgh - hVEGF-A heparin-binding domain - NMR
1qty, 1flt – hVEGF-A receptor-binding domain + hFMS-like tyrosine
1vpp – hVEGF-A + receptor blocking peptide
1bj1 – hVEGF-A + mFab light+heavy chain fragments
2vpf, 1vpf – hVEGF-A receptor binding domain
1kat - hVEGF-A receptor binding domain + peptide antagonist - NMR
3s1b - hVEGF-A + Mini-Z
3s1k - hVEGF-A + Z-domain
4gln, 4gls - hVEGF-A + D-RFX001
4deq – hVEGF-A /neuropilin-1
VEGF-B
2xac – hVEGF-B receptor binding domain+ hVEGFR domain 2
2vwe – hVEGF-B + mFab light+heavy chain fragments
2c7w – hVEGF-B
3v2a – hVEGF-B + VEGF-A
VEGF-C
2x1w, 2x1x – hVEGF-C VEGF homology domain+ VEGFR-2 domains 2 & 3
4bsk - hVEGF-C VEGF homology domain + VEGFR-3 domains 1 & 2
VEGF-D
2xv7 – hVEGF-D VEGF homology domain
VEGF-E
3v6b – VEGF-E + VEGFR 2 – Orf virus
VEGF-F
1wq8, 1wq9 - VEGF-F – Vipera aspis
Additional Resources
For additional information, see: Cancer
See Also
References
- ↑ Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983-5. PMID:6823562
- ↑ Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851-8. PMID:2735925
- ↑ Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004 Aug;25(4):581-611. PMID:15294883 doi:10.1210/er.2003-0027
- ↑ Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005 Jan 21;280(3):2126-31. Epub 2004 Nov 12. PMID:15542594 doi:10.1074/jbc.M411395200
- ↑ Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843-5. PMID:1279431 doi:http://dx.doi.org/10.1038/359843a0
- ↑ Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997 Feb;18(1):4-25. PMID:9034784
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435-9. PMID:8602241 doi:http://dx.doi.org/10.1038/380435a0
- ↑ Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001 Mar;114(Pt 5):853-65. PMID:11181169
- ↑ Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7192-7. PMID:9207067
- ↑ Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996 Mar 8;271(10):5638-46. PMID:8621427
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Oefner C, D'Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921-6. PMID:1396586
- ↑ Pieren M, Prota AE, Ruch C, Kostrewa D, Wagner A, Biedermann K, Winkler FK, Ballmer-Hofer K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J Biol Chem. 2006 Jul 14;281(28):19578-87. Epub 2006 May 3. PMID:16672228 doi:10.1074/jbc.M601842200
- ↑ Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. Identification of placenta growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem. 2004 Oct 15;279(42):43929-39. Epub 2004 Jul 21. PMID:15272021 doi:10.1074/jbc.M401418200
- ↑ Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004 Oct;30(5):427-46. PMID:15488020 doi:10.1111/j.1365-2990.2004.00600.x
- ↑ Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol. 2010 Jun 17. PMID:20662002 doi:10.1002/path.2748
- ↑ http://www.foxbusiness.com/story/markets/industries/technology/roche-increased-avastin-sales-efforts-doubles-force/
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Wayne Decatur, Jaime Prilusky