Potassium channel Xavier
From Proteopedia
(Difference between revisions)
| Line 32: | Line 32: | ||
At this point it is clear to see where the channels remarkable selectivity comes from. | At this point it is clear to see where the channels remarkable selectivity comes from. | ||
When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters of the <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene>. | When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters of the <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene>. | ||
| + | |||
| + | Another factor that drives the cations leave the aqueous cavity and enter the selectivity filter is | ||
| + | the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing at the entrance of the selectivity filter</scene> | ||
===== Why K+ and not Na+ ===== | ===== Why K+ and not Na+ ===== | ||
To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. | To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. | ||
| + | |||
| + | |||
===== Knock on and polarity of helices ===== | ===== Knock on and polarity of helices ===== | ||
| - | There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate. | + | There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate. , helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> |
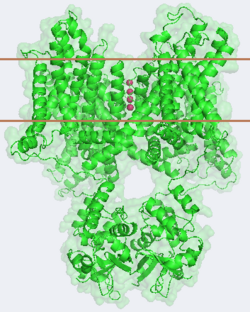
It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | ||
Revision as of 18:40, 13 September 2014
--Xavier Prat-Resina 21:22, 13 September 2014 (IDT)Important: This is a modified version by Xavier Prat-Resina of the original article on Potassium Channel. The purpose of this modification is simplifying the text and reversing the direction of potassium pumping. Any credits should be given to the authors of the Potassium Channel page.
Structure and mechanism of the potassium channel
Overview of structure
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedLong - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDoyle