Potassium channel Xavier
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
==== Entering the hydrophobic pocket ==== | ==== Entering the hydrophobic pocket ==== | ||
It is important to notice that with the exception of the selectivity filter, the pore lining is <scene name='Potassium_Channel/Full_pore_hdryo/2'>mainly hydrophobic</scene>. | It is important to notice that with the exception of the selectivity filter, the pore lining is <scene name='Potassium_Channel/Full_pore_hdryo/2'>mainly hydrophobic</scene>. | ||
| - | The entrance of the channel, at the bottom of the 34Å pore containing transmembrane region lies a number of <scene name='Potassium_Channel/High_filter_aromatic/2'>aromatic residues</scene> which help form a seal between the pore and the intracellular cytoplasm. | + | The entrance of the channel, at the bottom of the 34Å pore containing transmembrane region lies a number of <scene name='Potassium_Channel/High_filter_aromatic/2'>aromatic residues</scene> which help form a seal between the pore and the intracellular cytoplasm. This domain is called the lower gate. |
==== Aqueous cavity and desolvation ==== | ==== Aqueous cavity and desolvation ==== | ||
| - | This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired. At the end of the hydrophobic porus there is an <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene> (<scene name='Potassium_Channel/Full_pore_h20_spin/1'>Spinning Model</scene>). At this point, K<sup>+</sup> ions find a position of hydration and get ready to be dehydrated and get into the selectivity filter. | + | This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired, without getting attached anywhere until the aqueous cavity. At the end of the hydrophobic porus there is an <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene> (<scene name='Potassium_Channel/Full_pore_h20_spin/1'>Spinning Model</scene>). At this point, K<sup>+</sup> ions find a position of hydration and get ready to be dehydrated and get into the selectivity filter. |
====Selectivity Filter and Pore==== | ====Selectivity Filter and Pore==== | ||
| Line 33: | Line 33: | ||
When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters of the <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene>. | When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters of the <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene>. | ||
| - | Another factor that drives the cations leave the aqueous cavity and enter the selectivity filter is | + | Another factor that drives the cations leave the aqueous cavity and enter the selectivity filter is the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing at the entrance of the selectivity filter</scene> |
| - | + | ||
===== Why K+ and not Na+ ===== | ===== Why K+ and not Na+ ===== | ||
| Line 41: | Line 40: | ||
| + | ===== Knock-on mechanism ===== | ||
| + | There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | ||
| - | ==== | + | ==== Closing and opening ==== |
| - | + | ||
| + | ===== Hydrophobic gating: Dewetting ===== | ||
| + | |||
| + | ===== Physical gating: The Voltage Sensor Domain (VSD) ===== | ||
It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | ||
Revision as of 02:26, 14 September 2014
--Xavier Prat-Resina 21:22, 13 September 2014 (IDT)Important: This is a modified version by Xavier Prat-Resina of the original article on Potassium Channel. The purpose of this modification is simplifying the text and reversing the direction of potassium pumping. Any credits should be given to the authors of the Potassium Channel page.
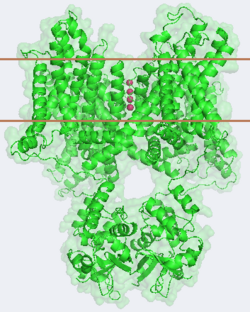
Structure and mechanism of the potassium channel
Overview of structure
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedLong - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDoyle