We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1r73
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
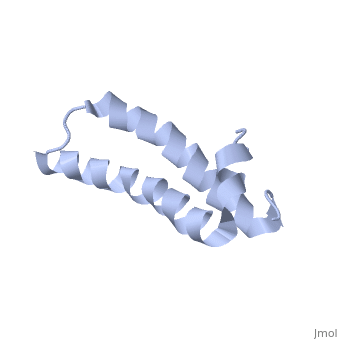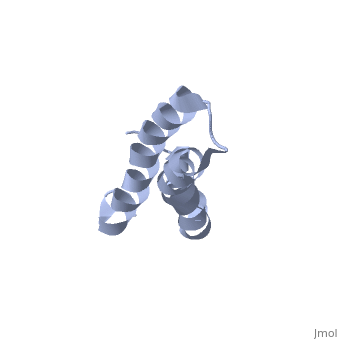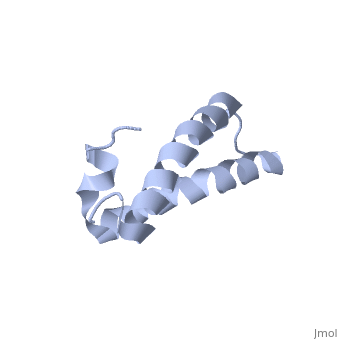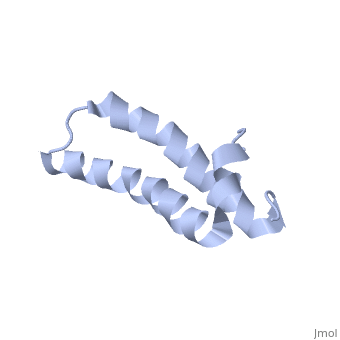
| - | [[ | + | ==Solution Structure of TM1492, the L29 ribosomal protein from Thermotoga maritima== |
| + | <StructureSection load='1r73' size='340' side='right' caption='[[1r73]], [[NMR_Ensembles_of_Models | 20 NMR models]]' scene=''> | ||
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[1r73]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Thermotoga_maritima Thermotoga maritima]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1R73 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1R73 FirstGlance]. <br> | ||
| + | </td></tr><tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1r73 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1r73 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1r73 RCSB], [http://www.ebi.ac.uk/pdbsum/1r73 PDBsum], [http://www.topsan.org/Proteins/JCSG/1r73 TOPSAN]</span></td></tr> | ||
| + | <table> | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/r7/1r73_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | This paper describes the NMR screening of 141 small (<15 kDa) recombinant Thermotoga maritima proteins for globular folding. The experimental data shows that approximately 25% of the screened proteins are folded under our screening conditions, which makes this procedure an important step for selecting those proteins that are suitable for structure determination. A comparison of screening based either on 1D 1H NMR with unlabeled proteins or on 2D [1H,15N]-COSY with uniformly 15N-labeled proteins is presented, and a comprehensive analysis of the 1D 1H NMR screening data is described. As an illustration of the utility of these methods to structural proteomics, the NMR structure determination of TM1492 (ribosomal protein L29) is presented. This 66-residue protein consists of a N-terminal 3(10)-helix and two long alpha-helices connected by a tight turn centered about glycine 35, where conserved leucine and isoleucine residues in the two alpha-helices form a small hydrophobic core. | ||
| - | + | NMR for structural proteomics of Thermotoga maritima: screening and structure determination.,Peti W, Etezady-Esfarjani T, Herrmann T, Klock HE, Lesley SA, Wuthrich K J Struct Funct Genomics. 2004;5(3):205-15. PMID:15263836<ref>PMID:15263836</ref> | |
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==See Also== | ==See Also== | ||
*[[Ribosomal protein L29|Ribosomal protein L29]] | *[[Ribosomal protein L29|Ribosomal protein L29]] | ||
| - | + | == References == | |
| - | == | + | <references/> |
| - | < | + | __TOC__ |
| + | </StructureSection> | ||
[[Category: Thermotoga maritima]] | [[Category: Thermotoga maritima]] | ||
[[Category: Etezady-Esfarjani, T.]] | [[Category: Etezady-Esfarjani, T.]] | ||
Revision as of 00:14, 29 September 2014
Solution Structure of TM1492, the L29 ribosomal protein from Thermotoga maritima
| |||||||||||