This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2km6
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | [[ | + | ==NMR structure of the NLRP7 Pyrin domain== |
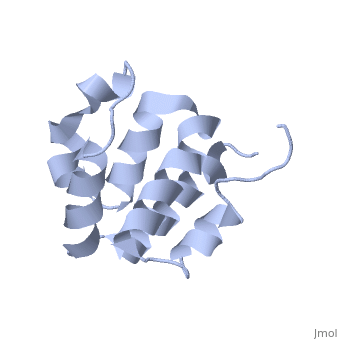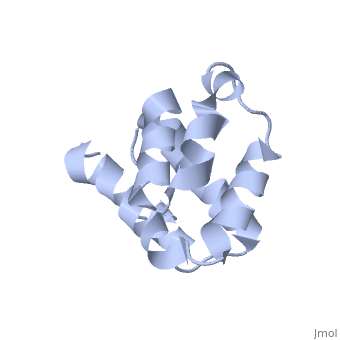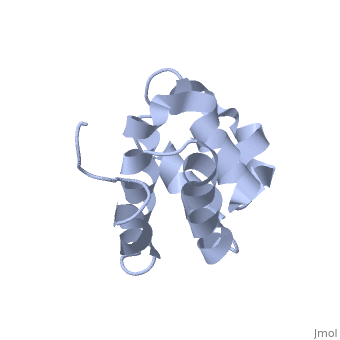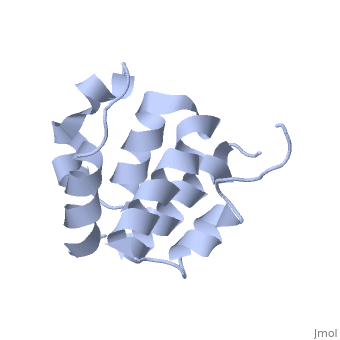
| + | <StructureSection load='2km6' size='340' side='right' caption='[[2km6]], [[NMR_Ensembles_of_Models | 20 NMR models]]' scene=''> | ||
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[2km6]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2KM6 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2KM6 FirstGlance]. <br> | ||
| + | </td></tr><tr><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">NLRP7, NALP7, NOD12, PYPAF3 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> | ||
| + | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2km6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2km6 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2km6 RCSB], [http://www.ebi.ac.uk/pdbsum/2km6 PDBsum]</span></td></tr> | ||
| + | <table> | ||
| + | == Disease == | ||
| + | [[http://www.uniprot.org/uniprot/NALP7_HUMAN NALP7_HUMAN]] Partial hydatidiform mole;Complete hydatidiform mole. The disease is caused by mutations affecting the gene represented in this entry.<ref>PMID:16462743</ref> <ref>PMID:19246479</ref> | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/NALP7_HUMAN NALP7_HUMAN]] Inhibits CASP1/caspase-1-dependent IL1B secretion.<ref>PMID:15817483</ref> | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/km/2km6_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | The innate immune system provides an initial line of defense against infection. Nucleotide-binding domain- and leucine-rich repeat-containing protein (NLR or (NOD-like)) receptors play a critical role in the innate immune response by surveying the cytoplasm for traces of intracellular invaders and endogenous stress signals. NLRs themselves are multi-domain proteins. Their N-terminal effector domains (typically a pyrin or caspase activation and recruitment domain) are responsible for driving downstream signaling and initiating the formation of inflammasomes, multi-component complexes necessary for cytokine activation. However, the currently available structures of NLR effector domains have not yet revealed the mechanism of their differential modes of interaction. Here, we report the structure and dynamics of the N-terminal pyrin domain of NLRP7 (NLRP7 PYD) obtained by NMR spectroscopy. The NLRP7 PYD adopts a six-alpha-helix bundle death domain fold. A comparison of conformational and dynamics features of the NLRP7 PYD with other PYDs showed distinct differences for helix alpha3 and loop alpha2-alpha3, which, in NLRP7, is stabilized by a strong hydrophobic cluster. Moreover, the NLRP7 and NLRP1 PYDs have different electrostatic surfaces. This is significant, because death domain signaling is driven by electrostatic contacts and stabilized by hydrophobic interactions. Thus, these results provide new insights into NLRP signaling and provide a first molecular understanding of inflammasome formation. | ||
| - | + | Three-dimensional structure of the NLRP7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity.,Pinheiro AS, Proell M, Eibl C, Page R, Schwarzenbacher R, Peti W J Biol Chem. 2010 Aug 27;285(35):27402-10. Epub 2010 Jun 11. PMID:20547486<ref>PMID:20547486</ref> | |
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==See Also== | ==See Also== | ||
*[[Pyrin domain|Pyrin domain]] | *[[Pyrin domain|Pyrin domain]] | ||
| - | + | == References == | |
| - | == | + | <references/> |
| - | < | + | __TOC__ |
| + | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Peti, W.]] | [[Category: Peti, W.]] | ||
Revision as of 03:59, 29 September 2014
NMR structure of the NLRP7 Pyrin domain
| |||||||||||