1hdz
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:1hdz.gif|left|200px]] | + | [[Image:1hdz.gif|left|200px]] |
| - | + | ||
| - | '''THREE-DIMENSIONAL STRUCTURES OF THREE HUMAN ALCOHOL DEHYDROGENASE VARIANTS: CORRELATIONS WITH THEIR FUNCTIONAL DIFFERENCES''' | + | {{Structure |
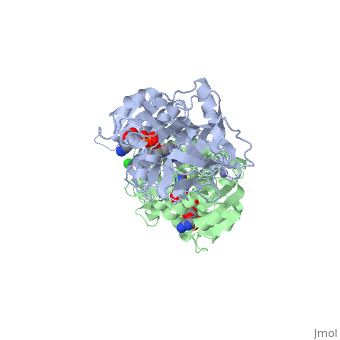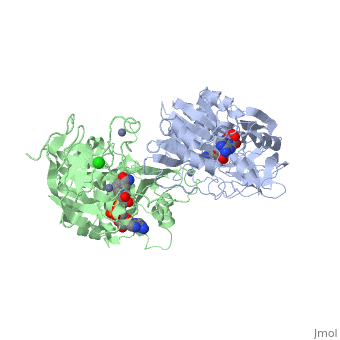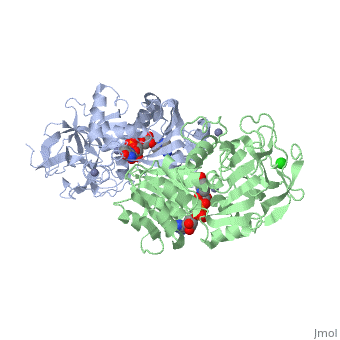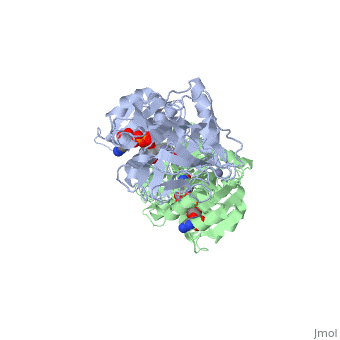
| + | |PDB= 1hdz |SIZE=350|CAPTION= <scene name='initialview01'>1hdz</scene>, resolution 2.5Å | ||
| + | |SITE= | ||
| + | |LIGAND= <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene>, <scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene> and <scene name='pdbligand=NAD:NICOTINAMIDE-ADENINE-DINUCLEOTIDE'>NAD</scene> | ||
| + | |ACTIVITY= [http://en.wikipedia.org/wiki/Alcohol_dehydrogenase Alcohol dehydrogenase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.1.1.1 1.1.1.1] | ||
| + | |GENE= | ||
| + | }} | ||
| + | |||
| + | '''THREE-DIMENSIONAL STRUCTURES OF THREE HUMAN ALCOHOL DEHYDROGENASE VARIANTS: CORRELATIONS WITH THEIR FUNCTIONAL DIFFERENCES''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 10: | Line 19: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1HDZ is a [ | + | 1HDZ is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1HDZ OCA]. |
==Reference== | ==Reference== | ||
| - | Structures of three human beta alcohol dehydrogenase variants. Correlations with their functional differences., Hurley TD, Bosron WF, Stone CL, Amzel LM, J Mol Biol. 1994 Jun 10;239(3):415-29. PMID:[http:// | + | Structures of three human beta alcohol dehydrogenase variants. Correlations with their functional differences., Hurley TD, Bosron WF, Stone CL, Amzel LM, J Mol Biol. 1994 Jun 10;239(3):415-29. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8201622 8201622] |
[[Category: Alcohol dehydrogenase]] | [[Category: Alcohol dehydrogenase]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| Line 24: | Line 33: | ||
[[Category: oxidoreductase(nad(a)-choh(d))]] | [[Category: oxidoreductase(nad(a)-choh(d))]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 11:35:53 2008'' |
Revision as of 09:35, 20 March 2008
| |||||||
| , resolution 2.5Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , and | ||||||
| Activity: | Alcohol dehydrogenase, with EC number 1.1.1.1 | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
THREE-DIMENSIONAL STRUCTURES OF THREE HUMAN ALCOHOL DEHYDROGENASE VARIANTS: CORRELATIONS WITH THEIR FUNCTIONAL DIFFERENCES
Contents |
Overview
The three-dimensional structures of three variants of human beta alcohol dehydrogenase have been determined to 2.5 A resolution. These three structures differ only in the amino acid at position 47 and the molecules occupying the alcohol binding site. Human beta 1 alcohol dehydrogenase has an Arg at position 47 and was crystallized in a complex with NAD(H) and cyclohexanol. A naturally occurring variant of beta 1 alcohol dehydrogenase, found in approximately 50% of the Asian population, possesses a His at position 47 (beta 2 or beta 47H) and was crystallized in a complex with NAD+ and the inhibitor 4-iodopyrazole. A site-directed mutant of beta 1 alcohol dehydrogenase in which a Gly is substituted for Arg47 (beta 47G) was crystallized in a complex with NAD+. By comparing both the common and unique features of these structures, it is clear that position 47 contributes significantly to the strength of protein-coenzyme interactions. The substitution of Arg47 by His produces an enzyme with a 100-fold lower affinity for coenzyme, but creates no large changes in the enzyme structure. The substitution of Arg47 by Gly produces an enzyme with coenzyme binding characteristics more similar to the wild-type enzyme than to the enzyme with His at position 47, but the structure of the Gly47 variant exhibits differences in and around the coenzyme binding site. These changes involve a rigid-body rotation of the catalytic domain towards the coenzyme domain by approximately 0.8 degrees and local rearrangements of amino acid side-chains, such as a 1.0 A movement of Lys228, relative to the beta 1 enzyme. These structural alterations may compensate for the loss of coenzyme interactions contributed by Arg47 and can explain the high affinity of the Gly47 variant for coenzyme.
Disease
Known diseases associated with this structure: Alcoholism, susceptibility to OMIM:[103720]
About this Structure
1HDZ is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Structures of three human beta alcohol dehydrogenase variants. Correlations with their functional differences., Hurley TD, Bosron WF, Stone CL, Amzel LM, J Mol Biol. 1994 Jun 10;239(3):415-29. PMID:8201622
Page seeded by OCA on Thu Mar 20 11:35:53 2008