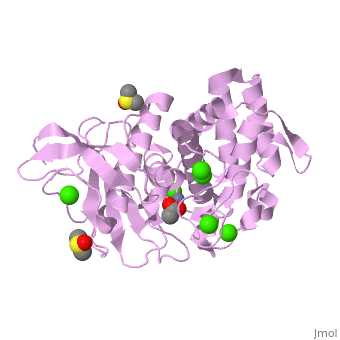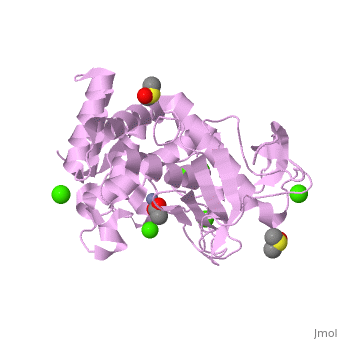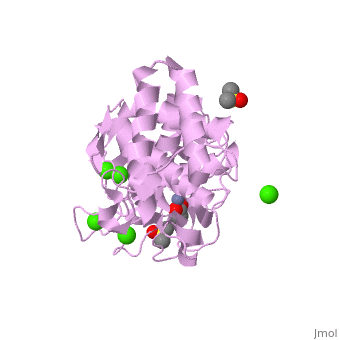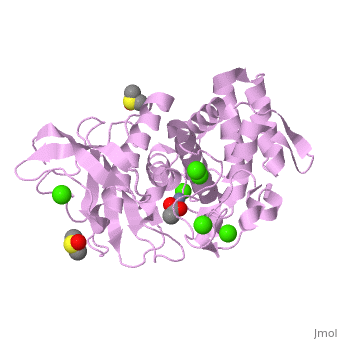This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2a7g
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ==On the Routine Use of Soft X-Rays in Macromolecular Crystallography, Part III- The Optimal Data Collection Wavelength== | |
| - | + | <StructureSection load='2a7g' size='340' side='right' caption='[[2a7g]], [[Resolution|resolution]] 1.85Å' scene=''> | |
| - | + | == Structural highlights == | |
| + | <table><tr><td colspan='2'>[[2a7g]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Bacillus_thermoproteolyticus Bacillus thermoproteolyticus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2A7G OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2A7G FirstGlance]. <br> | ||
| + | </td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ACY:ACETIC+ACID'>ACY</scene>, <scene name='pdbligand=CA:CALCIUM+ION'>CA</scene>, <scene name='pdbligand=DMS:DIMETHYL+SULFOXIDE'>DMS</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene><br> | ||
| + | <tr><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Transaldolase Transaldolase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.2.1.2 2.2.1.2] </span></td></tr> | ||
| + | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2a7g FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2a7g OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2a7g RCSB], [http://www.ebi.ac.uk/pdbsum/2a7g PDBsum]</span></td></tr> | ||
| + | <table> | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/a7/2a7g_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | Complete and highly redundant data sets were collected at different wavelengths between 0.80 and 2.65 A for a total of ten different protein and DNA model systems. The magnitude of the anomalous signal-to-noise ratio as assessed by the quotient R(anom)/R(r.i.m.) was found to be influenced by the data-collection wavelength and the nature of the anomalously scattering substructure. By utilizing simple empirical correlations, for instance between the estimated deltaF/F and the expected R(anom) or the data-collection wavelength and the expected R(r.i.m.), the wavelength at which the highest anomalous signal-to-noise ratio can be expected could be estimated even before the experiment. Almost independent of the nature of the anomalously scattering substructure and provided that no elemental X-ray absorption edge is nearby, this optimal wavelength is 2.1 A. | ||
| - | + | On the routine use of soft X-rays in macromolecular crystallography. Part III. The optimal data-collection wavelength.,Mueller-Dieckmann C, Panjikar S, Tucker PA, Weiss MS Acta Crystallogr D Biol Crystallogr. 2005 Sep;61(Pt 9):1263-72. Epub 2005, Aug 16. PMID:16131760<ref>PMID:16131760</ref> | |
| - | + | ||
| + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| + | </div> | ||
==See Also== | ==See Also== | ||
*[[Metalloproteases|Metalloproteases]] | *[[Metalloproteases|Metalloproteases]] | ||
*[[Thermolysin|Thermolysin]] | *[[Thermolysin|Thermolysin]] | ||
| - | + | == References == | |
| - | == | + | <references/> |
| - | < | + | __TOC__ |
| + | </StructureSection> | ||
[[Category: Bacillus thermoproteolyticus]] | [[Category: Bacillus thermoproteolyticus]] | ||
[[Category: Transaldolase]] | [[Category: Transaldolase]] | ||
Revision as of 03:00, 30 September 2014
On the Routine Use of Soft X-Rays in Macromolecular Crystallography, Part III- The Optimal Data Collection Wavelength
| |||||||||||