2p1q
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ==Mechanism of Auxin Perception by the TIR1 ubiquitin ligase== | |
| - | === | + | <StructureSection load='2p1q' size='340' side='right' caption='[[2p1q]], [[Resolution|resolution]] 1.91Å' scene=''> |
| - | + | == Structural highlights == | |
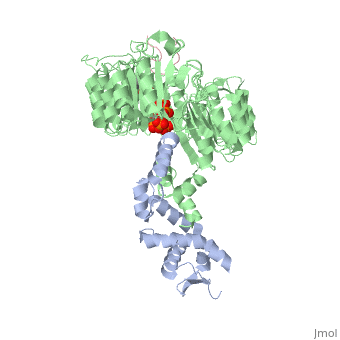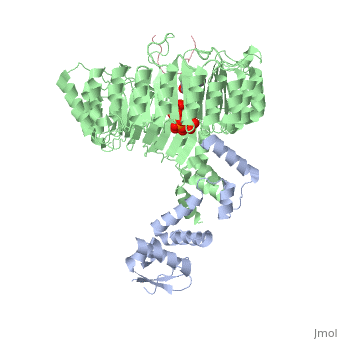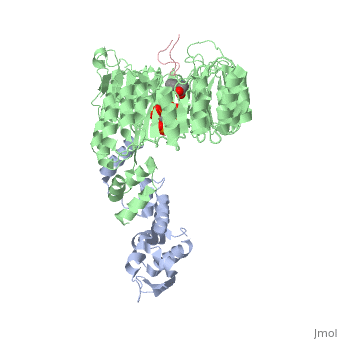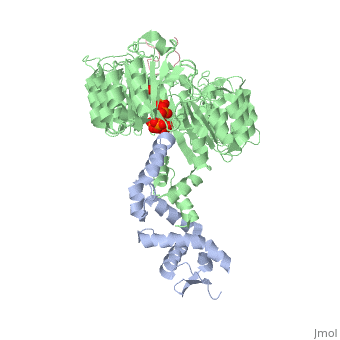
| + | <table><tr><td colspan='2'>[[2p1q]] is a 3 chain structure with sequence from [http://en.wikipedia.org/wiki/Arabidopsis_thaliana Arabidopsis thaliana]. The February 2009 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Auxin and TIR1 Ubiquitin Ligase'' by David Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2009_2 10.2210/rcsb_pdb/mom_2009_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P1Q OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2P1Q FirstGlance]. <br> | ||
| + | </td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=IAC:1H-INDOL-3-YLACETIC+ACID'>IAC</scene>, <scene name='pdbligand=IHP:INOSITOL+HEXAKISPHOSPHATE'>IHP</scene><br> | ||
| + | <tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2p1m|2p1m]], [[2p1n|2p1n]], [[2p1o|2p1o]], [[2p1p|2p1p]]</td></tr> | ||
| + | <tr><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SKP1A, ASK1, SKP1, UIP1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=3702 Arabidopsis thaliana]), TIR1, FBL1, WEI1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=3702 Arabidopsis thaliana])</td></tr> | ||
| + | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2p1q FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2p1q OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2p1q RCSB], [http://www.ebi.ac.uk/pdbsum/2p1q PDBsum]</span></td></tr> | ||
| + | <table> | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/p1/2p1q_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | Auxin is a pivotal plant hormone that controls many aspects of plant growth and development. Perceived by a small family of F-box proteins including transport inhibitor response 1 (TIR1), auxin regulates gene expression by promoting SCF ubiquitin-ligase-catalysed degradation of the Aux/IAA transcription repressors, but how the TIR1 F-box protein senses and becomes activated by auxin remains unclear. Here we present the crystal structures of the Arabidopsis TIR1-ASK1 complex, free and in complexes with three different auxin compounds and an Aux/IAA substrate peptide. These structures show that the leucine-rich repeat domain of TIR1 contains an unexpected inositol hexakisphosphate co-factor and recognizes auxin and the Aux/IAA polypeptide substrate through a single surface pocket. Anchored to the base of the TIR1 pocket, auxin binds to a partially promiscuous site, which can also accommodate various auxin analogues. Docked on top of auxin, the Aux/IAA substrate peptide occupies the rest of the TIR1 pocket and completely encloses the hormone-binding site. By filling in a hydrophobic cavity at the protein interface, auxin enhances the TIR1-substrate interactions by acting as a 'molecular glue'. Our results establish the first structural model of a plant hormone receptor. | ||
| - | + | Mechanism of auxin perception by the TIR1 ubiquitin ligase.,Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N Nature. 2007 Apr 5;446(7136):640-5. PMID:17410169<ref>PMID:17410169</ref> | |
| - | + | ||
| + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| + | </div> | ||
==See Also== | ==See Also== | ||
*[[TIR1 ubiquitin ligase|TIR1 ubiquitin ligase]] | *[[TIR1 ubiquitin ligase|TIR1 ubiquitin ligase]] | ||
*[[Transport inhibitor response 1|Transport inhibitor response 1]] | *[[Transport inhibitor response 1|Transport inhibitor response 1]] | ||
| - | + | *[[Ubiquitin protein ligase|Ubiquitin protein ligase]] | |
| - | == | + | == References == |
| - | < | + | <references/> |
| + | __TOC__ | ||
| + | </StructureSection> | ||
[[Category: Arabidopsis thaliana]] | [[Category: Arabidopsis thaliana]] | ||
[[Category: Auxin and TIR1 Ubiquitin Ligase]] | [[Category: Auxin and TIR1 Ubiquitin Ligase]] | ||
Revision as of 20:03, 30 September 2014
Mechanism of Auxin Perception by the TIR1 ubiquitin ligase
| |||||||||||