Sandbox Dicer Corey
From Proteopedia
| Line 5: | Line 5: | ||
== History == | == History == | ||
| - | Dicer, or endoribonuclease Dicer, was discovered/named in 2001 by Emily Bernstein. She was a graduate student in Greg Hannon's lab at the Cold Spring Harbor Laboratory in New York. She was trying to discover the enzyme that was responsible for removing small RNA fragments from double-stranded RNA. The dicer enzyme was found by isolating it from the RISC complex in the RNAi mechanism. It was known that RISC was not responsible for chopping up these small RNA fragments, so this complex was isolated from the system to locate the enzyme that was the source for these RNA fragments. | + | Dicer, or endoribonuclease Dicer, was discovered/named in 2001 by Emily Bernstein. She was a graduate student in Greg Hannon's lab at the Cold Spring Harbor Laboratory in New York. She was trying to discover the enzyme that was responsible for removing small RNA fragments from double-stranded RNA. The dicer enzyme was found by isolating it from the RISC complex in the RNAi mechanism. It was known that RISC was not responsible for chopping up these small RNA fragments, so this complex was isolated from the system to locate the enzyme that was the source for these RNA fragments.(1) |
| - | Dicer is a member of the RNase III family, is known as drosophilia CG4792 and it is found in multiple organisms. The discovery of Dicer was important for understanding the regulation of gene expression and the epigenetic silencing of genes by miRNA. The human endoribonuclease Dicer is 219 kDa, which is larger than many other organisms' Dicer enzymes. This is due to Humans having different domains present, and in many cases, more domains. | + | Dicer is a member of the RNase III family, is known as drosophilia CG4792 and it is found in multiple organisms.(2,3) The discovery of Dicer was important for understanding the regulation of gene expression and the epigenetic silencing of genes by miRNA. The human endoribonuclease Dicer is 219 kDa, which is larger than many other organisms' Dicer enzymes. This is due to Humans having different domains present, and in many cases, more domains.(3) |
== Structure and Mechanism == | == Structure and Mechanism == | ||
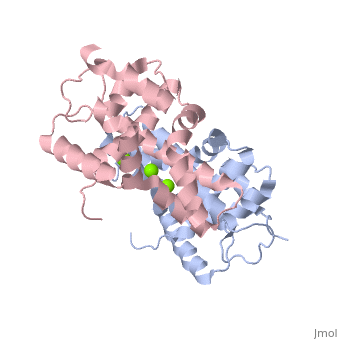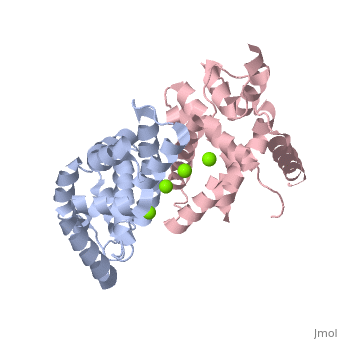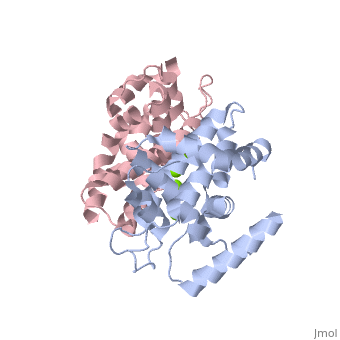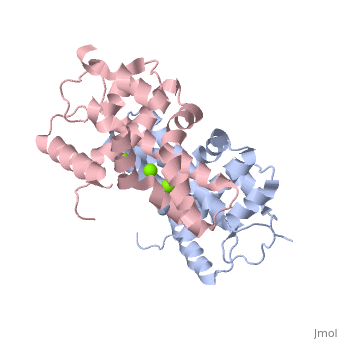
| - | Human Dicer is made up of helicase and PAZ domains in addition to two RNaseIII domains, A and B, and two double stranded RNA binding domains. The PAZ domain binds the 3' overhang of the dsRNA, while the two dsRNA binding domains bind to the dsRNA to hold the strand in place. At the same time, RNaseIIIa and RNaseIIIb form a dimer, and bind to the dsRNA via the active site. The active site is made up of a combination of the two RNaseIII domains. Both domains contain two Mg2+ ions which each stabilize two glutamine residues and two aspartic acid residues. <scene name='60/602707/Active_site/2'>Active Site</scene> These combine to form the two halves of the active site which allow the two RNaseIII domains to form the pseudo-dimer around the dsRNA. <scene name='60/602707/Trench/2'>pseudodimer</scene> Between the 3' end of the dsRNA which is bound to the PAZ domain, and the 5' end, which is bound to the PAZ domain loop. This allows for 25 nucleotides to be between the 3' binding site and the RNaseIIIa domain, which is then cleaved to form the RNA fragment. In this mechanism, the Dicer acts as a 25 nucleotide long ruler. | + | Human Dicer is made up of helicase and PAZ domains in addition to two RNaseIII domains, A and B, and two double stranded RNA binding domains. The PAZ domain binds the 3' overhang of the dsRNA, while the two dsRNA binding domains bind to the dsRNA to hold the strand in place. At the same time, RNaseIIIa and RNaseIIIb form a dimer, and bind to the dsRNA via the active site. The active site is made up of a combination of the two RNaseIII domains. Both domains contain two Mg2+ ions which each stabilize two glutamine residues and two aspartic acid residues. <scene name='60/602707/Active_site/2'>Active Site</scene> These combine to form the two halves of the active site which allow the two RNaseIII domains to form the pseudo-dimer around the dsRNA. <scene name='60/602707/Trench/2'>pseudodimer</scene> Between the 3' end of the dsRNA which is bound to the PAZ domain, and the 5' end, which is bound to the PAZ domain loop. This allows for 25 nucleotides to be between the 3' binding site and the RNaseIIIa domain, which is then cleaved to form the RNA fragment. In this mechanism, the Dicer acts as a 25 nucleotide long ruler.(2) |
== Importance == | == Importance == | ||
| - | The Human Dicer and its mechanism is of the utmost importance because it cleaves these dsRNA's to form small interfering RNA or microRNA, which are then integrated into the RNA-induced silencing complex, or the RISC complex. This complex then targets mRNA and prevents translation by disrupting the targeted gene. Without Dicer, gene silencing cannot occur. Therefore, without Dicer, DNA and RNA cannot be regulated. | + | The Human Dicer and its mechanism is of the utmost importance because it cleaves these dsRNA's to form small interfering RNA or microRNA, which are then integrated into the RNA-induced silencing complex, or the RISC complex.(2,4) This complex then targets mRNA and prevents translation by disrupting the targeted gene. Without Dicer, gene silencing cannot occur. Therefore, without Dicer, DNA and RNA cannot be regulated. |
== Diseases == | == Diseases == | ||
| - | Dicer is known to be a direct cause of macular degeneration. The abscence of Dicer in retinal pigment epithelium causes the eye to break down into macular degeneration. It is hypothesized that Dicer has a specific role in maintaining this retinal health. | + | Dicer is known to be a direct cause of macular degeneration. The abscence of Dicer in retinal pigment epithelium causes the eye to break down into macular degeneration. It is hypothesized that Dicer has a specific role in maintaining this retinal health.(5) |
| - | In cancer cells, especially in lung and ovarian cancers, low dicer levels allow for malignant cells to duplicate and develop. In prostate and esophageal cancers, high dicer counts are a direct correlation to poor patient prognosis. Dicer's role is considered to be unique in different cancer types because of these relations. | + | In cancer cells, especially in lung and ovarian cancers, low dicer levels allow for malignant cells to duplicate and develop. In prostate and esophageal cancers, high dicer counts are a direct correlation to poor patient prognosis. Dicer's role is considered to be unique in different cancer types because of these relations.(5) |
</StructureSection> | </StructureSection> | ||
Current revision
Introduction
| |||||||||||
References
1. Bernstein, E, Caudy, A, Hammond, S, and Hannon, G. (2001) Role for a Bidentate ribonuclease in the initial step of RNA interference. Cold Spring Harbor Laboratory. Nature, Vol 409, pgs. 363-367
2. MacRae, I. (2006) Structural Basis for Double-Stranded RNA processing by Dicer. Science, Vol 311, pgs. 195-198
3. Hammond, S. (2005) Dicing and Slicing: The Core machinery of the RNA interference pathway. University of North Carolina. Federation of European Biochemical Societies, Letters 579, pgs. 5822-5829
4. Khaiwesh, B, Asif Arif, M, Seumel, G, Ossowski, S, Weigel, D, Reski, R, and Frank, W. (2010) Transcriptional control of gene expression by microRNAs. Cell, Vol 140, pgs. 111-122
5. Lau, P, Potter, C, Carragher, B, MacRae, I. (2009) Structure of the Human dicer-TRBP complex by Electron Microscopy. Cell, October 14, 2009, pgs. 1326-1332.