We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
P53-DNA Recognition
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='3kz8bio-4mon.pdb.zip' size='400' side='right' scene='' caption=''> | + | <StructureSection load='3kz8bio-4mon.pdb.zip' size='400' side='right' ' oldscene='Sandbox_Reserved_170/Complex/6' scene='P53-DNA_Recognition/P53_complex/1' caption=''> |
''This is a joint project of students at La Cañada High School, La Cañada Flintridge, California USA, and students at the University of Southern California, Los Angeles, California USA, mentored by [[User:Remo Rohs|Professor Remo Rohs]].'' | ''This is a joint project of students at La Cañada High School, La Cañada Flintridge, California USA, and students at the University of Southern California, Los Angeles, California USA, mentored by [[User:Remo Rohs|Professor Remo Rohs]].'' | ||
| Line 22: | Line 22: | ||
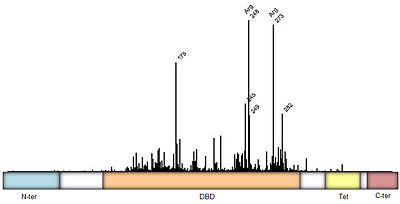
===Domain Architecture and Tetramerization=== | ===Domain Architecture and Tetramerization=== | ||
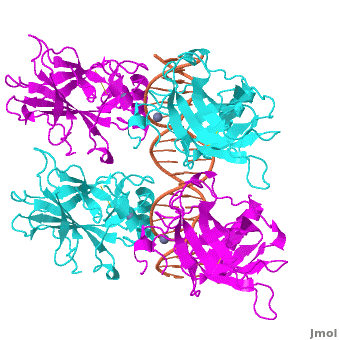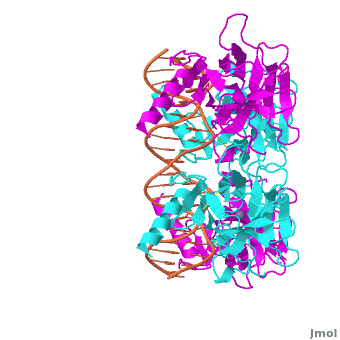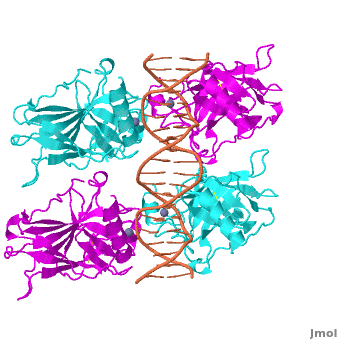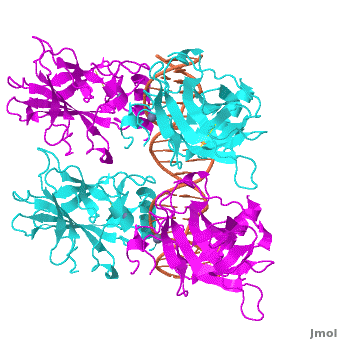
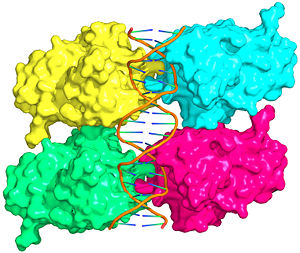
| - | The p53 protein consists of the N-terminal transactivation domain, the DNA binding domain ('''DBD''') or core, the tetramerization domain ([[#Tetramerization Domain|see its structure below]]), and the C-terminal regulatory domain ('''Figure 3'''). This Proteopedia page discusses protein-DNA recognition by p53, thus focusing on the DBD of p53 (<scene name='P53-DNA_Recognition/P53_complex/1'>Figure 4: Crystal structure of p53 DBD tetramer-DNA complex</scene>, [[3kz8|PDB ID 3KZ8]]). | + | The p53 protein consists of the N-terminal transactivation domain, the DNA binding domain ('''DBD''') or core, the tetramerization domain ([[#Tetramerization Domain|see its structure below]]), and the C-terminal regulatory domain ('''Figure 3'''). This Proteopedia page discusses protein-DNA recognition by p53, thus focusing on the DBD of p53 (<scene oldname='Sandbox_Reserved_170/Complex/6' name='P53-DNA_Recognition/P53_complex/1'>Figure 4: Crystal structure of p53 DBD tetramer-DNA complex</scene>, [[3kz8|PDB ID 3KZ8]]). |
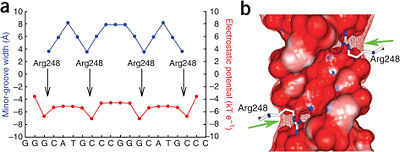
The DBD in tetrameric form binds to a <font color="#e06000">'''DNA response element'''</font> (<scene oldname='Sandbox_Reserved_170/Complex/6' name='P53-DNA_Recognition/P53_complex/1'>restore initial scene</scene>), which consists of two DNA half sites. These decameric half sites can be separated by a DNA spacer of flexible length but in this case, the spacer is of length zero base pairs. The <scene oldname='Sandbox_Reserved_170/Complex/7' name='P53-DNA_Recognition/P53_complex/2'>p53 tetramer binds DNA as a dimer of dimers</scene> with each <font color='e000e0'>'''magenta'''</font>-<font color='00c0c0'>'''cyan'''</font> dimer binding to one half site of the response element<ref>Kitayner M, Rozenberg H, Kessler N, Rabinovich D, Shaulov L, Haran TE, Shakked Z. Structural basis of DNA recognition by p53 tetramers. Mol Cell. 2006 Jun 23;22(6):741-53. [http://www.ncbi.nlm.nih.gov/pubmed/16793544 PMID:16793544].</ref>. | The DBD in tetrameric form binds to a <font color="#e06000">'''DNA response element'''</font> (<scene oldname='Sandbox_Reserved_170/Complex/6' name='P53-DNA_Recognition/P53_complex/1'>restore initial scene</scene>), which consists of two DNA half sites. These decameric half sites can be separated by a DNA spacer of flexible length but in this case, the spacer is of length zero base pairs. The <scene oldname='Sandbox_Reserved_170/Complex/7' name='P53-DNA_Recognition/P53_complex/2'>p53 tetramer binds DNA as a dimer of dimers</scene> with each <font color='e000e0'>'''magenta'''</font>-<font color='00c0c0'>'''cyan'''</font> dimer binding to one half site of the response element<ref>Kitayner M, Rozenberg H, Kessler N, Rabinovich D, Shaulov L, Haran TE, Shakked Z. Structural basis of DNA recognition by p53 tetramers. Mol Cell. 2006 Jun 23;22(6):741-53. [http://www.ncbi.nlm.nih.gov/pubmed/16793544 PMID:16793544].</ref>. | ||
Revision as of 12:39, 2 November 2014
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Remo Rohs, Eric Martz, Alexander Berchansky, Julia Tam, Sharon Kim, Bailey Holmes, Angel Herraez, Joseph M. Steinberger, Eran Hodis, Masha Karelina, Michal Harel, Ana Carolina Dantas Machado, Jaime Prilusky, Skyler Saleebyan, Joel L. Sussman, Keziah Kim