1kcg
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:1kcg.gif|left|200px]] | + | [[Image:1kcg.gif|left|200px]] |
| - | + | ||
| - | '''NKG2D in complex with ULBP3''' | + | {{Structure |
| + | |PDB= 1kcg |SIZE=350|CAPTION= <scene name='initialview01'>1kcg</scene>, resolution 2.6Å | ||
| + | |SITE= | ||
| + | |LIGAND= <scene name='pdbligand=GSH:GLUTATHIONE'>GSH</scene> | ||
| + | |ACTIVITY= | ||
| + | |GENE= | ||
| + | }} | ||
| + | |||
| + | '''NKG2D in complex with ULBP3''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1KCG is a [ | + | 1KCG is a [[Protein complex]] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1KCG OCA]. |
==Reference== | ==Reference== | ||
| - | Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3., Radaev S, Rostro B, Brooks AG, Colonna M, Sun PD, Immunity. 2001 Dec;15(6):1039-49. PMID:[http:// | + | Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3., Radaev S, Rostro B, Brooks AG, Colonna M, Sun PD, Immunity. 2001 Dec;15(6):1039-49. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/11754823 11754823] |
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
| Line 20: | Line 29: | ||
[[Category: protein-protein complex]] | [[Category: protein-protein complex]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 12:15:29 2008'' |
Revision as of 10:15, 20 March 2008
| |||||||
| , resolution 2.6Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
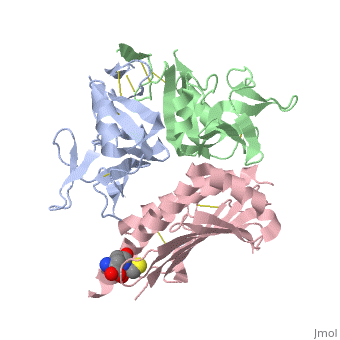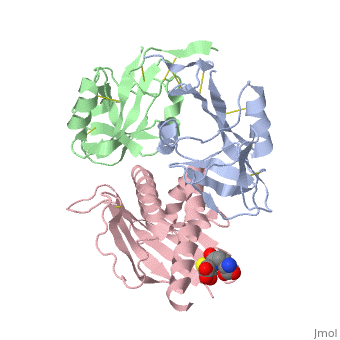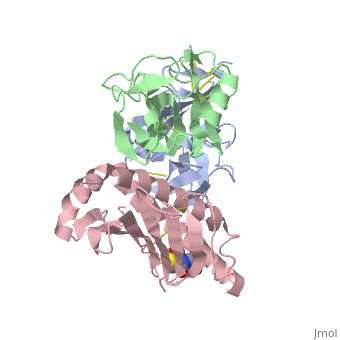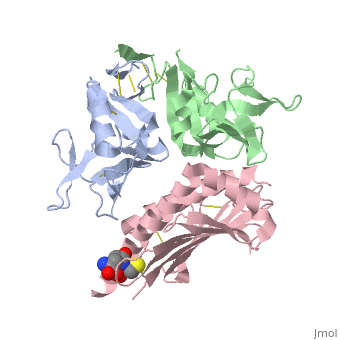
NKG2D in complex with ULBP3
Overview
NKG2D is known to trigger the natural killer (NK) cell lysis of various tumor and virally infected cells. In the NKG2D/ULBP3 complex, the structure of ULBP3 resembles the alpha1 and alpha2 domains of classical MHC molecules without a bound peptide. The lack of alpha3 and beta2m domains is compensated by replacing two hydrophobic patches at the underside of the class I MHC-like beta sheet floor with a group of hydrophilic and charged residues in ULBP3. NKG2D binds diagonally across the ULBP3 alpha helices, creating a complementary interface, an asymmetrical subunit orientation, and local conformational adjustments in the receptor. The interface is stabilized primarily by hydrogen bonds and hydrophobic interactions. Unlike the KIR receptors that recognize a conserved HLA region by a lock-and-key mechanism, NKG2D recognizes diverse ligands by an induced-fit mechanism.
About this Structure
1KCG is a Protein complex structure of sequences from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3., Radaev S, Rostro B, Brooks AG, Colonna M, Sun PD, Immunity. 2001 Dec;15(6):1039-49. PMID:11754823
Page seeded by OCA on Thu Mar 20 12:15:29 2008