We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Telomerase
From Proteopedia
(Difference between revisions)
m (Sandbox Telomerase Jackson Stevens moved to Telomerase: original Sandbox by Jackson Stevens) |
|||
| Line 1: | Line 1: | ||
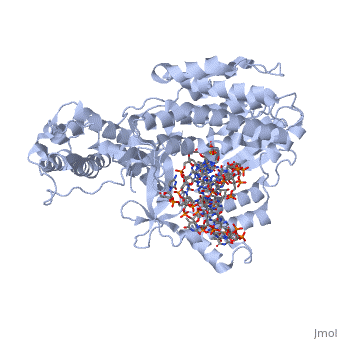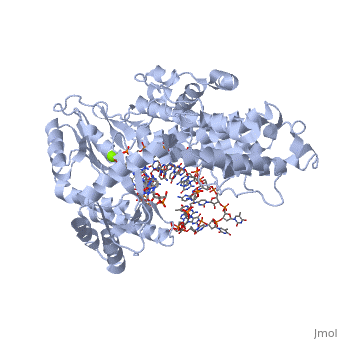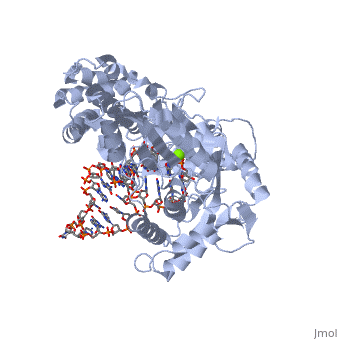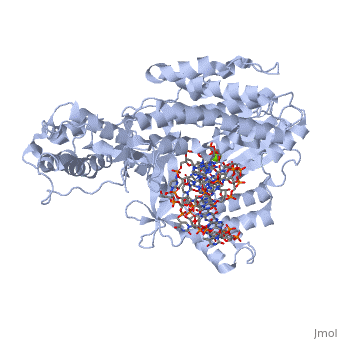
| - | < | + | <StructureSection load='3kyl' size='350' side='right' caption='Telomerase: bound to telomeric DNA [[3kyl]]' scene=''> |
| - | + | ||
== Introduction == | == Introduction == | ||
The telomerase is a complex composed of an RNA primer and protein that adds G-rich repeated DNA sequences to a 3' DNA strand in eukaryotic cells (1). This ribonucleoprotein acts as a reverse transcriptase that creates a DNA sequence complementary to its RNA primer in its catalytic subunit, TERT (2). The sequences attached to the end of DNA is non-coding DNA that functions as protection from mutation and degradation. As DNA replications occurs, the polymerase that copies in the 5' to 3' direction, cannot copy the end of the lagging strand which leaves unpaired bases that may code for a specific gene (3). Multiple unpaired ends can form hydrogen bonds or even swap genetic material. The telomerase fixes this issue and lengthens the strands, because the replication process slowly shortens the ends of chromosomes. This enzyme also plays a role in coordinating cell division. Complementary issues of aging and cancer describe the inactivation or over-activation of telomerase activity (3). | The telomerase is a complex composed of an RNA primer and protein that adds G-rich repeated DNA sequences to a 3' DNA strand in eukaryotic cells (1). This ribonucleoprotein acts as a reverse transcriptase that creates a DNA sequence complementary to its RNA primer in its catalytic subunit, TERT (2). The sequences attached to the end of DNA is non-coding DNA that functions as protection from mutation and degradation. As DNA replications occurs, the polymerase that copies in the 5' to 3' direction, cannot copy the end of the lagging strand which leaves unpaired bases that may code for a specific gene (3). Multiple unpaired ends can form hydrogen bonds or even swap genetic material. The telomerase fixes this issue and lengthens the strands, because the replication process slowly shortens the ends of chromosomes. This enzyme also plays a role in coordinating cell division. Complementary issues of aging and cancer describe the inactivation or over-activation of telomerase activity (3). | ||
| Line 26: | Line 25: | ||
The reverse transcription process begins by the telomerase RNA binding to the 3' end of the overhanging DNA sequence. Once primed, the G-rich sequence (~6-8 nucleotides) polymerizes the end of the DNA. The RNA primer then translocates down the new DNA sequence and polymerizes again. This process repeats until the entire telomere is formed. After one side of the telomere is formed, the telomerase attaches the complementary nucleotide bases to complete the double-stranded telomere at the end of the DNA double helix. | The reverse transcription process begins by the telomerase RNA binding to the 3' end of the overhanging DNA sequence. Once primed, the G-rich sequence (~6-8 nucleotides) polymerizes the end of the DNA. The RNA primer then translocates down the new DNA sequence and polymerizes again. This process repeats until the entire telomere is formed. After one side of the telomere is formed, the telomerase attaches the complementary nucleotide bases to complete the double-stranded telomere at the end of the DNA double helix. | ||
| + | |||
| + | ==See Also== | ||
| + | [[Telomerase 3D structures]] | ||
== References == | == References == | ||
| Line 38: | Line 40: | ||
5. Mitchell, Meghan, Andrew Gillis, Mizuko Futahashi, Haruhiko Fujiwara, and Emmanuel Skordalakes. "Structural Basis for Telomerase Catalytic Subunit TERT Binding to RNA Template and Telomeric DNA." Nature Structural & Molecular Biology 17.4 (2010): 513-18. Web. | 5. Mitchell, Meghan, Andrew Gillis, Mizuko Futahashi, Haruhiko Fujiwara, and Emmanuel Skordalakes. "Structural Basis for Telomerase Catalytic Subunit TERT Binding to RNA Template and Telomeric DNA." Nature Structural & Molecular Biology 17.4 (2010): 513-18. Web. | ||
| + | |||
| + | </StructureSection> | ||
Revision as of 04:39, 6 November 2014
| |||||||||||