RA Mediated T-reg Differentiation
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
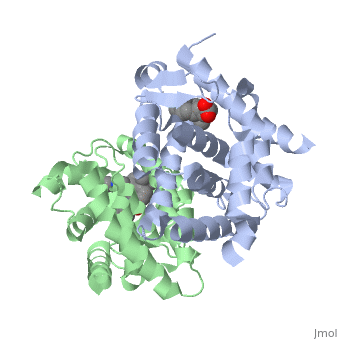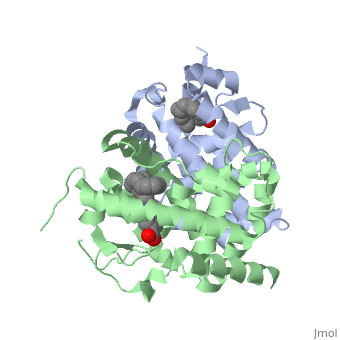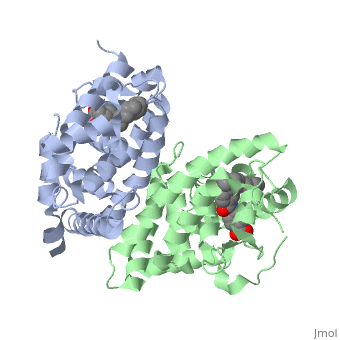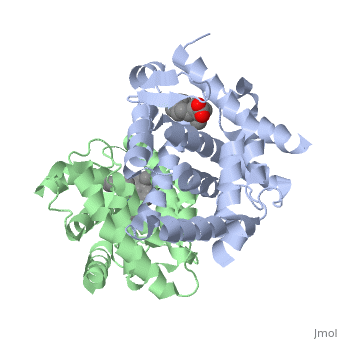
The <scene name='RA_Mediated_T-reg_Differentiaition/Rxr-ligand_binding_pocket/1'>RXR-alpha binding pocket</scene> is comprised of 16 primarily hydrophobic residues, found on the H3, H5, H7, H11, and L11-12 domains. The ligand used in the crystal, Oleic Acid, is similar to RA, and RA is capable of binding to the RXRα pocket.<ref> PMID: 10882070 </ref> | The <scene name='RA_Mediated_T-reg_Differentiaition/Rxr-ligand_binding_pocket/1'>RXR-alpha binding pocket</scene> is comprised of 16 primarily hydrophobic residues, found on the H3, H5, H7, H11, and L11-12 domains. The ligand used in the crystal, Oleic Acid, is similar to RA, and RA is capable of binding to the RXRα pocket.<ref> PMID: 10882070 </ref> | ||
| - | |||
| - | </StructureSection> | ||
| - | <StructureSection load='1BY4' size='350' side='right' caption='Crystal structure of RXRα-DNA complex (PDB entry [[1by4]])' scene=''> | ||
==DNA Binding Domain== | ==DNA Binding Domain== | ||
| + | <scene name='51/519788/Cv/2'>Crystal structure of RXRα-DNA complex</scene> (PDB entry [[1by4]]). | ||
When RXRα homodimers assemble on DNA, they form a four poplypeptide complex assembled via head to tail interactions along DR-1 repeated sequences. The | When RXRα homodimers assemble on DNA, they form a four poplypeptide complex assembled via head to tail interactions along DR-1 repeated sequences. The | ||
<scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_alpha_helices/1'>alpha helical</scene> structures of the polypeptides sit in the major grooves of the DNA chain, allowing for interaction with specific bases, giving a sequence specificity for the protein. The two <scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_zn_domains/2'>Zinc containing domains</scene> do not alter their configuration upon DNA binding, but are used to guide the DNA into the correct position. Upon binding to DNA, the C-terminal end of the protein, referred to as the <scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_t-box/1'> "T-box" </scene> alters its conformation from alpha helical to an extended conformation. This extended conformation allows Glu74 to move away from the DNA binding pocket and moves it so it interacts with the Zn(II) domain of the next polypeptide. <ref> PMID: 10669605 </ref> | <scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_alpha_helices/1'>alpha helical</scene> structures of the polypeptides sit in the major grooves of the DNA chain, allowing for interaction with specific bases, giving a sequence specificity for the protein. The two <scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_zn_domains/2'>Zinc containing domains</scene> do not alter their configuration upon DNA binding, but are used to guide the DNA into the correct position. Upon binding to DNA, the C-terminal end of the protein, referred to as the <scene name='RA_Mediated_T-reg_Differentiaition/Rxr_dbd_t-box/1'> "T-box" </scene> alters its conformation from alpha helical to an extended conformation. This extended conformation allows Glu74 to move away from the DNA binding pocket and moves it so it interacts with the Zn(II) domain of the next polypeptide. <ref> PMID: 10669605 </ref> | ||
| Line 48: | Line 46: | ||
=== DR-5 Specificity === | === DR-5 Specificity === | ||
RXRα/RARα homodimers have not yet been crystallized on DNA, but have been shown to associate with extended DR-5 tandem repeats. The sequence of this DR-5 repeat is AGGTCA-nnnnn-AGGTCA. Additonally, when incubated with retinoic acid, cells expressing RARα showed high expression of genes located downstream of DR-5 tandem repeats. <ref> PMID: 1648450 </ref> | RXRα/RARα homodimers have not yet been crystallized on DNA, but have been shown to associate with extended DR-5 tandem repeats. The sequence of this DR-5 repeat is AGGTCA-nnnnn-AGGTCA. Additonally, when incubated with retinoic acid, cells expressing RARα showed high expression of genes located downstream of DR-5 tandem repeats. <ref> PMID: 1648450 </ref> | ||
| - | + | ||
==Biological Significance== | ==Biological Significance== | ||
Since T-regulatory cells are so highly regulated in the body, elucidating the exact mechanism of activation can show how these immune processes work, and use them in the treatment of disease. Two clear mechanisms of regulation arise from the studies, both which are related to the heterodimer itself. First, the ligand specificity for RA in both molecules allows for specific signaling of these molecules. RA is not normally expressed in cells, and therefore will limit when this heterodimer is activated. Likewise, the propensity for the heterodimer to associate with DR-5 repeats limits the number of genes it will activate to a select few. All of this in addition to the other cytokines necessary, TGF-β and IL-2, show the complex mechanisms regulating the differentiation of T-helper cells into T-regulatory cells. | Since T-regulatory cells are so highly regulated in the body, elucidating the exact mechanism of activation can show how these immune processes work, and use them in the treatment of disease. Two clear mechanisms of regulation arise from the studies, both which are related to the heterodimer itself. First, the ligand specificity for RA in both molecules allows for specific signaling of these molecules. RA is not normally expressed in cells, and therefore will limit when this heterodimer is activated. Likewise, the propensity for the heterodimer to associate with DR-5 repeats limits the number of genes it will activate to a select few. All of this in addition to the other cytokines necessary, TGF-β and IL-2, show the complex mechanisms regulating the differentiation of T-helper cells into T-regulatory cells. | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
<references /> | <references /> | ||
Revision as of 13:06, 24 November 2014
| |||||||||||
References
- ↑ Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009 May;123(5):977-83; quiz 984-5. PMID:19410687 doi:10.1016/j.jaci.2009.03.030
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999 May;64(5):310-9. PMID:10406480
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F. Structural basis of RXR-DNA interactions. J Mol Biol. 2000 Feb 18;296(2):509-20. PMID:10669605 doi:10.1006/jmbi.1999.3457
- ↑ Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F. Structural basis of RXR-DNA interactions. J Mol Biol. 2000 Feb 18;296(2):509-20. PMID:10669605 doi:10.1006/jmbi.1999.3457
- ↑ Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255-66. PMID:1648450
Proteopedia Page Contributors and Editors (what is this?)
William Bailey, Alexander Berchansky, Michal Harel, Jaime Prilusky