Molecular Playground/E. coli ClpP
From Proteopedia
| Line 8: | Line 8: | ||
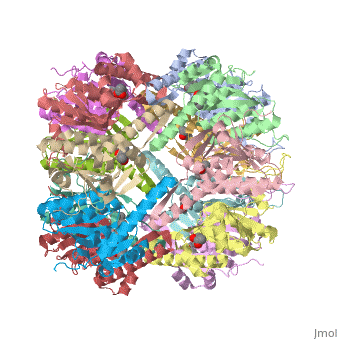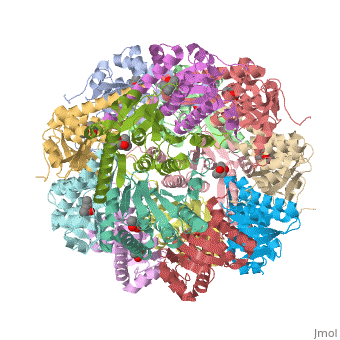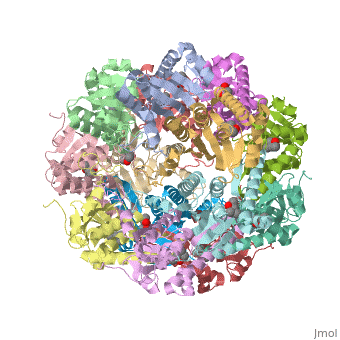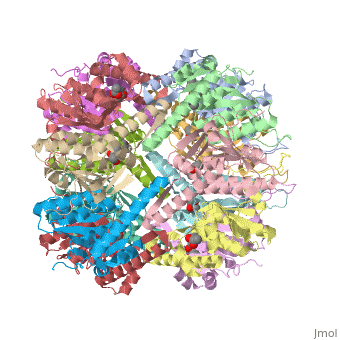
ClpP is a serine protease which consists of fourteen monomers situated into two [http://en.wiktionary.org/wiki/heptamer heptameric] rings seated on top of each other. In the center of the barrel-shaped chamber lies a core of fourteen peptide-cleaving active sites, restricted by the narrow entrance called the ''axial pore''. As peptides are [http://en.wikipedia.org/wiki/Processivity processively] threaded in the pore by the regulatory elements, the peptides are then degraded into smaller fragments as a result from ClpP cleavage. Smaller peptides are then released through small openings found around the equatorial interface of the two stacked rings. | ClpP is a serine protease which consists of fourteen monomers situated into two [http://en.wiktionary.org/wiki/heptamer heptameric] rings seated on top of each other. In the center of the barrel-shaped chamber lies a core of fourteen peptide-cleaving active sites, restricted by the narrow entrance called the ''axial pore''. As peptides are [http://en.wikipedia.org/wiki/Processivity processively] threaded in the pore by the regulatory elements, the peptides are then degraded into smaller fragments as a result from ClpP cleavage. Smaller peptides are then released through small openings found around the equatorial interface of the two stacked rings. | ||
== Structural highlights == | == Structural highlights == | ||
| - | Featured within the core of ClpP are the catalytic sets of amino acids [X-Y-Z] that constitute the active mechanism featured by serene proteases [http://www.ncbi.nlm.nih.gov/pubmed/2197276][ | + | Featured within the core of ClpP are the catalytic sets of amino acids [X-Y-Z] that constitute the active mechanism featured by serene proteases [http://www.ncbi.nlm.nih.gov/pubmed/2197276][http://www.ncbi.nlm.nih.gov/pubmed/17499722]. Human ClpP can exist in two forms, a single heptameric ring or as a double stack set of hepatmeric rings where the double stacked form (tetradecamer) is the active form [8]. Tetradecamer ClpP is a stable but not a rigid structure that can undergo several conformational forms when interacting with its regulatory elements. By doing so both stabilizes processivity of translocation by the regulatory elements and is thought to increase likelihood of exposure to the active sites resulting in timely, small peptide formation. |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| Line 29: | Line 29: | ||
[http://www.ncbi.nlm.nih.gov/pubmed/2197276 6. ''Clp P represents a unique family of serine proteases.'' Maurizi MR ''et. al'' (1990 J Biolchem)] | [http://www.ncbi.nlm.nih.gov/pubmed/2197276 6. ''Clp P represents a unique family of serine proteases.'' Maurizi MR ''et. al'' (1990 J Biolchem)] | ||
| - | [7. ClpP: a distinctive family of cylindrical energy-dependent serine proteases | + | [http://www.ncbi.nlm.nih.gov/pubmed/17499722 7. ''ClpP: a distinctive family of cylindrical energy-dependent serine proteases'' YuAY ''et. al'' (2007 FEBS Lett.)] |
| + | |||
[8.Human mitochondrial ClpP is a stable heptamer that assembles into a tetradecamer in the presence of ClpX | [8.Human mitochondrial ClpP is a stable heptamer that assembles into a tetradecamer in the presence of ClpX | ||
[9. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system | [9. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system | ||
Revision as of 20:38, 3 December 2014
Here in the Chien lab, we study how proteolysis plays a large part in protein quality control. The maintenance and timely destruction of protein levels plays an important role during cell homeostasis and cell transitions/differentiation, yet much of what governs these processes has yet to be fully understood.
| |||||||||||
References
6. Clp P represents a unique family of serine proteases. Maurizi MR et. al (1990 J Biolchem)
[8.Human mitochondrial ClpP is a stable heptamer that assembles into a tetradecamer in the presence of ClpX [9. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system [10. Bacterial Cell Stress Protein ClpP: A Novel Antibiotic Target [11. Homology in structural organization between E. coli ClpAP protease and the eukaryotic 26 S proteasome [12. Proteasomes and their kin: proteases in the machine age
Acknowledgements
Kamal Joshi, Joanne Lau, Jing Liu, Rob Vass