1n8z
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:1n8z.jpg|left|200px]] | + | [[Image:1n8z.jpg|left|200px]] |
| - | + | ||
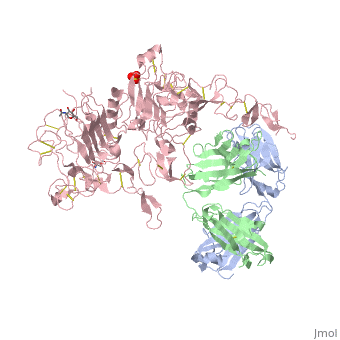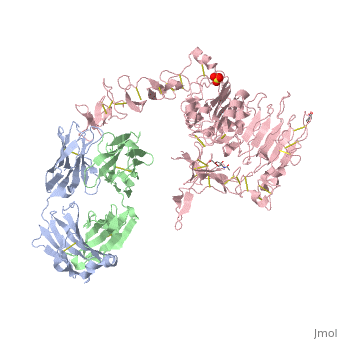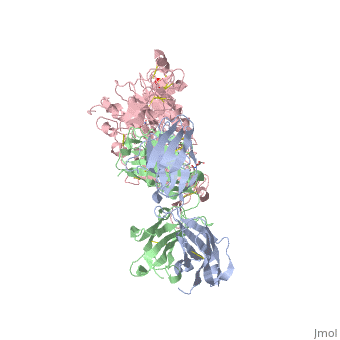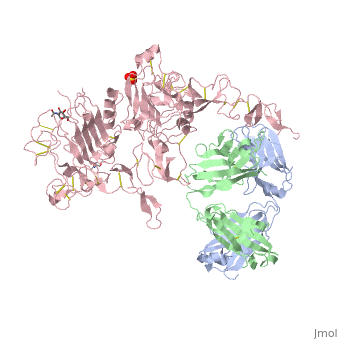
| - | '''Crystal structure of extracellular domain of human HER2 complexed with Herceptin Fab''' | + | {{Structure |
| + | |PDB= 1n8z |SIZE=350|CAPTION= <scene name='initialview01'>1n8z</scene>, resolution 2.52Å | ||
| + | |SITE= | ||
| + | |LIGAND= <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene> and <scene name='pdbligand=SO4:SULFATE ION'>SO4</scene> | ||
| + | |ACTIVITY= [http://en.wikipedia.org/wiki/Transferase Transferase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.10.1 and 2.7.10.2 2.7.10.1 and 2.7.10.2] | ||
| + | |GENE= HER2(erbB2) ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens]) | ||
| + | }} | ||
| + | |||
| + | '''Crystal structure of extracellular domain of human HER2 complexed with Herceptin Fab''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 10: | Line 19: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1N8Z is a [ | + | 1N8Z is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1N8Z OCA]. |
==Reference== | ==Reference== | ||
| - | Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab., Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ, Nature. 2003 Feb 13;421(6924):756-60. PMID:[http:// | + | Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab., Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ, Nature. 2003 Feb 13;421(6924):756-60. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/12610629 12610629] |
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| Line 29: | Line 38: | ||
[[Category: tyrosin kinase receptor]] | [[Category: tyrosin kinase receptor]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 12:53:43 2008'' |
Revision as of 10:53, 20 March 2008
| |||||||
| , resolution 2.52Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | and | ||||||
| Gene: | HER2(erbB2) (Homo sapiens) | ||||||
| Activity: | Transferase, with EC number and 2.7.10.2 2.7.10.1 and 2.7.10.2 | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Crystal structure of extracellular domain of human HER2 complexed with Herceptin Fab
Contents |
Overview
HER2 (also known as Neu, ErbB2) is a member of the epidermal growth factor receptor (EGFR; also known as ErbB) family of receptor tyrosine kinases, which in humans includes HER1 (EGFR, ERBB1), HER2, HER3 (ERBB3) and HER4 (ERBB4). ErbB receptors are essential mediators of cell proliferation and differentiation in the developing embryo and in adult tissues, and their inappropriate activation is associated with the development and severity of many cancers. Overexpression of HER2 is found in 20-30% of human breast cancers, and correlates with more aggressive tumours and a poorer prognosis. Anticancer therapies targeting ErbB receptors have shown promise, and a monoclonal antibody against HER2, Herceptin (also known as trastuzumab), is currently in use as a treatment for breast cancer. Here we report crystal structures of the entire extracellular regions of rat HER2 at 2.4 A and human HER2 complexed with the Herceptin antigen-binding fragment (Fab) at 2.5 A. These structures reveal a fixed conformation for HER2 that resembles a ligand-activated state, and show HER2 poised to interact with other ErbB receptors in the absence of direct ligand binding. Herceptin binds to the juxtamembrane region of HER2, identifying this site as a target for anticancer therapies.
Disease
Known diseases associated with this structure: Adenocarcinoma of lung, somatic OMIM:[164870], Gastric cancer, somatic OMIM:[164870], Glioblastoma, somatic OMIM:[164870], Ovarian cancer, somatic, OMIM:[164870], Sialidosis, type I OMIM:[608272], Sialidosis, type II OMIM:[608272]
About this Structure
1N8Z is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab., Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ, Nature. 2003 Feb 13;421(6924):756-60. PMID:12610629
Page seeded by OCA on Thu Mar 20 12:53:43 2008