Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
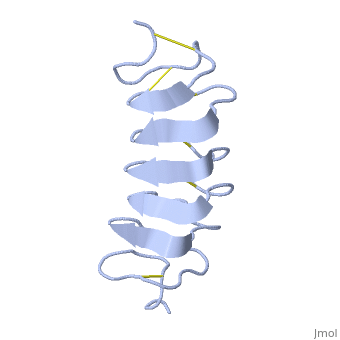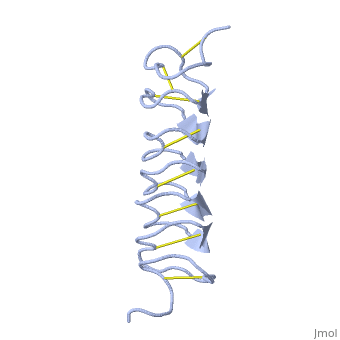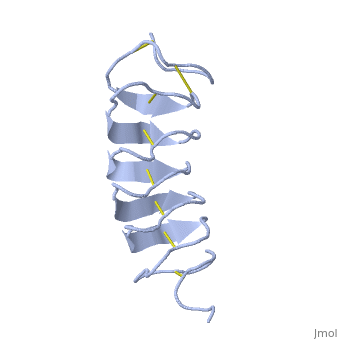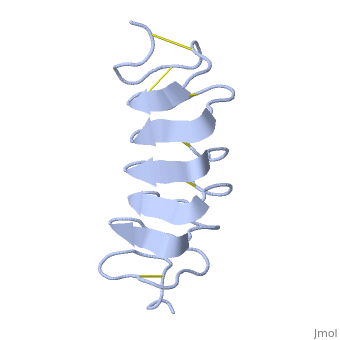
The rest of the tandem repeat forms the loop which enables very organized structure of the protein. | The rest of the tandem repeat forms the loop which enables very organized structure of the protein. | ||
| - | Cysteine all over the tandem repeats, provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein | + | Cysteine all over the tandem repeats, provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. |
| - | + | 6 of the 8 disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide</scene>2 disulphide bonds do not fit this pattern | |
| + | |||
Revision as of 09:44, 16 December 2014
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644