Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
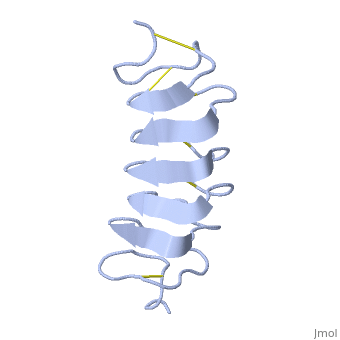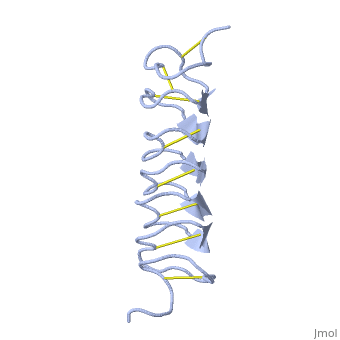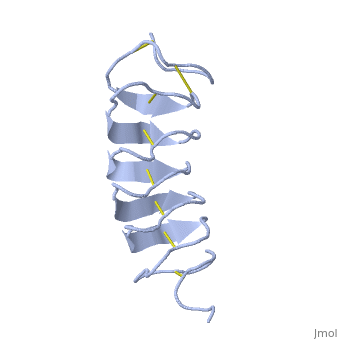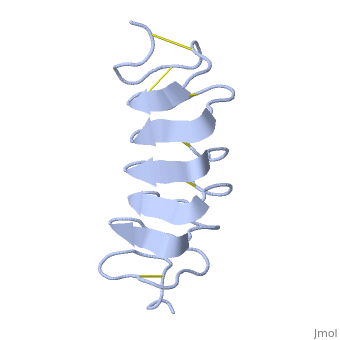
Cysteine all over the tandem repeats, provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. | Cysteine all over the tandem repeats, provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. | ||
| - | + | six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> do not fit this pattern. | |
| + | |||
| + | The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop hydrogen bond connections between backbone CO and NH groups. | ||
| + | |||
| + | TmAFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. | ||
| + | The few Hydrophobic residues <scene name='61/612804/Hydrophobic/1'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge. | ||
| + | |||
Revision as of 10:14, 16 December 2014
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644