1qjg
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Image:1qjg.jpg|left|200px]] | + | [[Image:1qjg.jpg|left|200px]] |
| - | + | ||
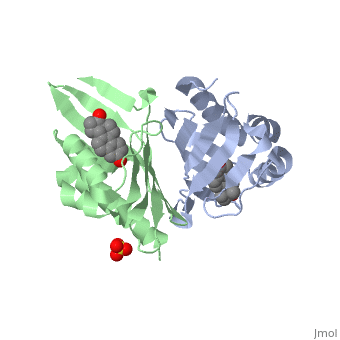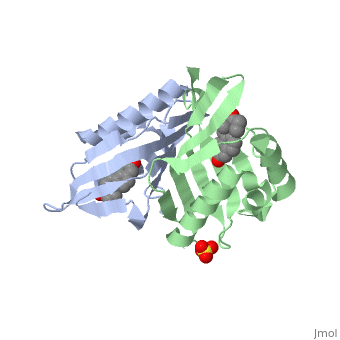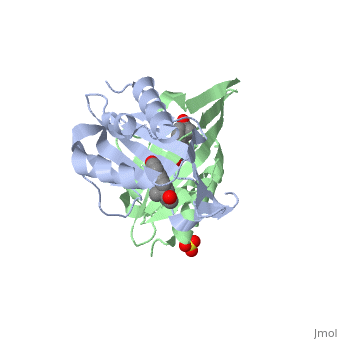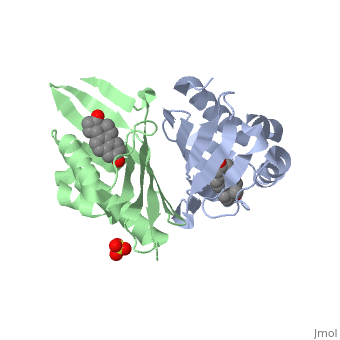
| - | '''CRYSTAL STRUCTURE OF DELTA5-3-KETOSTEROID ISOMERASE FROM PSEUDOMONAS TESTOSTERONI IN COMPLEX WITH EQUILENIN''' | + | {{Structure |
| + | |PDB= 1qjg |SIZE=350|CAPTION= <scene name='initialview01'>1qjg</scene>, resolution 2.3Å | ||
| + | |SITE= | ||
| + | |LIGAND= <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene> and <scene name='pdbligand=EQU:EQUILENIN'>EQU</scene> | ||
| + | |ACTIVITY= | ||
| + | |GENE= | ||
| + | }} | ||
| + | |||
| + | '''CRYSTAL STRUCTURE OF DELTA5-3-KETOSTEROID ISOMERASE FROM PSEUDOMONAS TESTOSTERONI IN COMPLEX WITH EQUILENIN''' | ||
| + | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1QJG is a [ | + | 1QJG is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Comamonas_testosteroni Comamonas testosteroni]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1QJG OCA]. |
==Reference== | ==Reference== | ||
| - | Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization., Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH, J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:[http:// | + | Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization., Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH, J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10551849 10551849] |
[[Category: Comamonas testosteroni]] | [[Category: Comamonas testosteroni]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| Line 21: | Line 30: | ||
[[Category: steroid isomeration]] | [[Category: steroid isomeration]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 13:38:29 2008'' |
Revision as of 11:38, 20 March 2008
| |||||||
| , resolution 2.3Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | and | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
CRYSTAL STRUCTURE OF DELTA5-3-KETOSTEROID ISOMERASE FROM PSEUDOMONAS TESTOSTERONI IN COMPLEX WITH EQUILENIN
Overview
Delta(5)-3-Ketosteroid isomerase from Pseudomonas testosteroni has been intensively studied as a prototype to understand an enzyme-catalyzed allylic isomerization. Asp(38) (pK(a) approximately 4.7) was identified as the general base abstracting the steroid C4beta proton (pK(a) approximately 12.7) to form a dienolate intermediate. A key and common enigmatic issue involved in the proton abstraction is the question of how the energy required for the unfavorable proton transfer can be provided at the active site of the enzyme and/or how the thermodynamic barrier can be drastically reduced. Answering this question has been hindered by the existence of two differently proposed enzyme reaction mechanisms. The 2.26 A crystal structure of the enzyme in complex with a reaction intermediate analogue equilenin reveals clearly that both the Tyr(14) OH and Asp(99) COOH provide direct hydrogen bonds to the oxyanion of equilenin. The result negates the catalytic dyad mechanism in which Asp(99) donates the hydrogen bond to Tyr(14), which in turn is hydrogen bonded to the steroid. A theoretical calculation also favors the doubly hydrogen-bonded system over the dyad system. Proton nuclear magnetic resonance analyses of several mutant enzymes indicate that the Tyr(14) OH forms a low barrier hydrogen bond with the dienolic oxyanion of the intermediate.
About this Structure
1QJG is a Single protein structure of sequence from Comamonas testosteroni. Full crystallographic information is available from OCA.
Reference
Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization., Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH, J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:10551849
Page seeded by OCA on Thu Mar 20 13:38:29 2008
Categories: Comamonas testosteroni | Single protein | Cho, H S. | Oh, B H. | EQU | SO4 | Isomerase | Ksi | Steroid isomeration